NUCLEAR GEOMETRY: from HYDROGEN to BORON Alexander I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Radiochemistry of Beryllium
National Academy of Sciences National Research Council I NUCLEAR SCIENCE SERIES The Radiochemistry ·of Beryllium COMMITTEE ON NUCLEAR SCIENCE L. F. CURTISS, Chairman ROBLEY D. EVANS, Vice Chairman National Bureau of Standards MassaChusetts Institute of Technol0gy J. A. DeJUREN, Secretary ./Westinghouse Electric Corporation H.J. CURTIS G. G. MANOV Brookhaven National' LaboratOry Tracerlab, Inc. SAMUEL EPSTEIN W. WAYNE MEINKE CalUornia Institute of Technology University of Michigan HERBERT GOLDSTEIN A.H. SNELL Nuclear Development Corporation of , oak Ridge National Laboratory America E. A. UEHLING H.J. GOMBERG University of Washington University of Michigan D. M. VAN PATTER E.D.KLEMA Bartol Research Foundation Northwestern University ROBERT L. PLATZMAN Argonne National Laboratory LIAISON MEMBERS PAUL C .. AEBERSOLD W.D.URRY Atomic Energy Commission U. S. Air Force J. HOW ARD McMILLEN WILLIAM E. WRIGHT National Science Foundation Office of Naval Research SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chairman HAROLD KIRBY University of Michigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE Florida State University. Oak Ridge National Laboratory GEORGE A. COW AN JULIAN NIELSEN Los Alamos Scientific Laboratory Hanford Laboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG University of Washington Argonne National Laboratory JEROME HUDIS PETER C. STEVENSON Brookhaven National Laboratory University of California (Livermore) EARL HYDE LEO YAFFE University of CalUornia (Berkeley) McGill University CONSULTANTS NATHAN BALLOU WILLIAM MARLOW Naval Radiological Defense Laboratory N atlonal Bureau of Standards JAMESDeVOE University of Michigan CHF.MISTRY-RADIATION AND RADK>CHEMIST The Radiochemistry of Beryllium By A. W. FAIRHALL. Department of Chemistry University of Washington Seattle, Washington May 1960 ' Subcommittee on Radiochemistry National Academy of Sciences - National Research Council Printed in USA. -

Measurement of 10Be from Lake Malawi (Africa) Drill Core Sediments and Implications for Geochronology
Palaeogeography, Palaeoclimatology, Palaeoecology 303 (2011) 110–119 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Measurement of 10Be from Lake Malawi (Africa) drill core sediments and implications for geochronology L.R. McHargue a,⁎, A.J.T. Jull b, A. Cohen a a Department of Geosciences, University of Arizona, 1040 E. Fourth Street, Tucson, Arizona 85721, United States b NSF Arizona AMS Laboratory, University of Arizona, 1118 E. Fourth Street, Tucson, Arizona 85721, United States article info abstract Article history: The cosmogenic radionuclide 10Be was measured from drill core sediments from Lake Malawi in order to Received 19 August 2008 help construct a chronology for the study of the tropical paleoclimate in East Africa. Sediment samples were Received in revised form 29 January 2010 taken every 10 m from the core MAL05-1C to 80 m in depth and then from that depth in core MAL05-1B to Accepted 4 February 2010 382 m. Sediment samples were then later taken at a higher resolution of every 2 m from MAL05-1C. They Available online 12 February 2010 were then leached to remove the authigenic fraction, the leachate was processed to separate out the beryllium isotopes, and 10Be was measured at the TAMS Facility at the University of Arizona. The 10Be/9Be Keywords: fi Beryllium-10 pro le from Lake Malawi sediments is similar to those derived from marine sediment cores for the late Cosmogenic radionuclide Pleistocene, and is consistent with the few radiocarbon and OSL IR measurements made from the same core. Lake sediments Nevertheless, a strong correlation between the stable isotope 9Be and the cosmogenic isotope 10Be suggests Lake Malawi that both isotopes have been well mixed before deposition unlike in some marine sediment cores. -

Lithium Isotope Fractionation During Uptake by Gibbsite
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 168 (2015) 133–150 www.elsevier.com/locate/gca Lithium isotope fractionation during uptake by gibbsite Josh Wimpenny a,⇑, Christopher A. Colla a, Ping Yu b, Qing-Zhu Yin a, James R. Rustad a, William H. Casey a,c a Department of Earth and Planetary Sciences, University of California, One Shields Avenue, Davis, CA 95616, United States b The Keck NMR Facility, University of California, One Shields Avenue, Davis, CA 95616, United States c Department of Chemistry, University of California, One Shields Avenue, Davis, CA 95616, United States Received 5 November 2014; accepted in revised form 8 July 2015; available online 15 July 2015 Abstract The intercalation of lithium from solution into the six-membered l2-oxo rings on the basal planes of gibbsite is well-constrained chemically. The product is a lithiated layered-double hydroxide solid that forms via in situ phase change. The reaction has well established kinetics and is associated with a distinct swelling of the gibbsite as counter ions enter the interlayer to balance the charge of lithiation. Lithium reacts to fill a fixed and well identifiable crystallographic site and has no solvation waters. Our lithium-isotope data shows that 6Li is favored during this intercalation and that the À À solid-solution fractionation depends on temperature, electrolyte concentration and counter ion identity (whether Cl ,NO3 À or ClO4 ). We find that the amount of isotopic fractionation between solid and solution (DLisolid-solution) varies with the amount of lithium taken up into the gibbsite structure, which itself depends upon the extent of conversion and also varies À À À with electrolyte concentration and in the counter ion in the order: ClO4 <NO3 <Cl . -

Online Determination of Boron Isotope Ratio in Boron Trifluoride By
applied sciences Article Online Determination of Boron Isotope Ratio in Boron Trifluoride by Infrared Spectroscopy Weijiang Zhang, Yin Tang and Jiao Xu * School of Chemical Engineering & Technology, Tianjin University, Tianjin 300072, China; [email protected] (W.Z.); [email protected] (Y.T.) * Correspondence: [email protected]; Tel.: +86-022-27402028 Received: 17 September 2018; Accepted: 3 December 2018; Published: 6 December 2018 Abstract: Enriched boron-10 and its related compounds have great application prospects, especially in the nuclear industry. The chemical exchange rectification method is one of the most important ways to separate the 10B and 11B isotope. However, a real-time monitoring method is needed because this separation process is difficult to characterize. Infrared spectroscopy was applied in the separation device to realize the online determination of the boron isotope ratio in boron trifluoride (BF3). The possibility of determining the isotope ratio via the 2ν3 band was explored. A correction factor was introduced to eliminate the difference between the ratio of peak areas and the true value of the boron isotope ratio. It was experimentally found that the influences of pressure and temperature could be ignored. The results showed that the infrared method has enough precision and stability for real-time, in situ determination of the boron isotope ratio. The instability point of the isotope ratio can be detected with the assistance of the online determination method and provides a reference for the production of boron isotope. Keywords: isotope; boron; infrared spectroscopy; online determination 1. Introduction Boron in naturally occurring compounds is composed of two isotopes, one of mass 10 and the other of mass 11. -

UNIT 1 SUMMARY MATTER – Anything That Has Mass and Takes up Space
UNIT 1 SUMMARY MATTER – Anything that has mass and takes up space Other examples? PARTICLE – a single atom or groups of atoms that are bonded together and function as one unit Matter is found in phases or states: GAS • Indefinite shape and volume • Straight-line motion LIQUID • Indefinite shape, definite volume • Rolling motion SOLID • Definite shape and volume • Vibrating motion TYPES OF MATTER PURE SUBSTANCE – Matter where all the particles are identical He NaCl C12H22O11 There are two types of pure substances: ELEMENT • Made from only one type of atom • Cannot be broken into simpler substances by chemical means • Found on the Periodic Table Other examples? Gold Sulfur Helium Sodium COMPOUND • Made from two or more atoms joined together by chemical bonds • Can be broken down into simpler substances by chemical means (by breaking chemical bonds) Water Salt Sugar Other examples? Water Compound ↓ electricity Hydrogen and Oxygen ↓ electricity ↓ electricity Hydrogen and Oxygen Elements Salt Compound ↓ electricity Sodium and Chlorine ↓ electricity ↓ electricity Sodium and Chlorine Elements In CHEMICAL SEPARATION METHODS (e.g. electrolysis), chemical bonds are broken and/or formed. MIXTURE – Matter with different types of particles (elements and/or compounds) There are two types of mixtures: HETEROGENEOUS – Has distinct parts with different properties HOMOGENEOUS – Has same properties throughout (uniform composition) Air Jell-O Steel Brass SOLUTION – A homogenous mixture CLASSIFICATION OF MATTER CHANGES IN MATTER PHYSICAL CHANGE – One that -

Meteoric Be and Be As Process Tracers in the Environment
Chapter 5 Meteoric 7Be and 10Be as Process Tracers in the Environment James M. Kaste and Mark Baskaran 7 10 Abstract Be (T1/2 ¼ 53 days) and Be (T1/2 ¼ occurring Be isotopes of use to Earth scientists are the 7 1.4 Ma) form via natural cosmogenic reactions in the short-lived Be (T1/2 ¼ 53.1 days) and the longer- 10 atmosphere and are delivered to Earth’s surface by wet lived Be (T1/2 ¼ 1.4 Ma; Nishiizumi et al. 2007). and dry deposition. The distinct source term and near- Because cosmic rays that cause the initial cascade of constant fallout of these radionuclides onto soils, vege- neutrons and protons in the upper atmosphere respon- tation, waters, ice, and sediments makes them valuable sible for the spallation reactions are attenuated by tracers of a wide range of environmental processes the mass of the atmosphere itself, production rates of operating over timescales from weeks to millions of comsogenic Be are three orders of magnitude higher in years. Beryllium tends to form strong bonds with oxygen the stratosphere than they are at sea-level (Masarik and atoms, so 7Be and 10Be adsorb rapidly to organic and Beer 1999, 2009). Most of the production of cosmo- inorganic solid phases in the terrestrial and marine envi- genic Be therefore occurs in the upper atmosphere ronment. Thus, cosmogenic isotopes of beryllium can be (5–30 km), although there is trace, but measurable used to quantify surface age, sediment source, mixing production as oxygen atoms in minerals at the Earth’s rates, and particle residence and transit times in soils, surface are spallated (in situ produced; see Lal 2011, streams, lakes, and the oceans. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Stable Isotopic Composition of Boron in Groundwater – Analytical
LLNL‐TR‐498360 LAWRENCE LIVERMORE NATIONAL LABORATORY California GAMA Special Study: Stable isotopic composition of boron in groundwater – analytical method development Gary R. Eppich, Josh B. Wimpenny*, Qingzhu Yin*, and Bradley K. Esser Lawrence Livermore National Laboratory *University of California, Davis October, 2011 Final report for the California State Water Resources Control Board GAMA Special Studies Task 10.4 : Development of New Wastewater Indicator Methods Disclaimer This document was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes. Auspices Statement This work performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE‐AC52‐07NA27344. GAMA: GROUNDWATER AMBIENT MONITORING & ASSESSMENT PROGRAM SPECIAL STUDY California GAMA Special Study: Stable isotopic composition of boron in groundwater – analytical method development By Gary R. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -

Name Midterm Review
NAME MIDTERM REVIEW 1. During a laboratory activity, a student combined two solutions. In the laboratory report, the student wrote “A yellow color appeared.” The statement represents the student’s recorded A) conclusion B) observation C) hypothesis D) inference 2. Which of the following statements contained in a student's laboratory report is a conclusion? A) A gas is evolved. B) The gas is insoluble in water. C) The gas is hydrogen. D) The gas burns in air. 3. The diagram below represents a portion of a 100-milliliter graduated cylinder. What is the reading of the meniscus? A) 35.0 mL B) 36.0 mL C) 44.0 mL D) 45.0 mL 4. An aluminum sample has a mass of 80.01 g and a density of 2.70 g/cm3. According to the data, to what number of significant figures should the calculated volume of the aluminum sample be expressed? A) 1 B) 2 C) 3 D) 4 5. A student in a laboratory determined the boiling point of a substance to be 71.8°C. The accepted value for the boiling point of this substance is 70.2°C. What is the percent error of the student's measurement? A) 1.60% B) 2.28% C) 2.23% D) 160.% MIDTERM REVIEW 6. Base your answer to the following question on the information below. A method used by ancient Egyptians to obtain copper metal from copper(I) sulfide ore was heating the ore in the presence of air. Later, copper was mixed with tin to produce a useful alloy called bronze. -
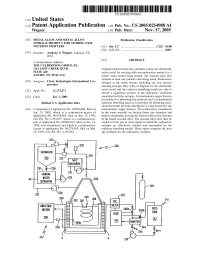
(12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub
US 2005O254988A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0254988A1 Wagner (43) Pub. Date: Nov. 17, 2005 (54) METALALLOY AND METALALLOY Publication Classification STORAGE PRODUCT FOR STORING EAST NEUTRON EMITTERS (51) Int. Cl. ................................................. C22C 43/00 (52) U.S. Cl. .................................................................. 420/1 (75) Inventor: Anthony S. Wagner, Lakeway, TX (US) (57) ABSTRACT Correspondence Address: THE CULBERTSON GROUP, PC. 1114 LOST CREEK BLVD. Aliquid reactant metal alloy includes at least one chemically SUTE 420 active metal for reacting with non-radioactive material in a AUSTIN, TX 78746 (US) mixed waste Stream being treated. The reactant alloy also includes at least one radiation absorbing metal. Radioactive (73) Assignee: Clean Technologies International Cor isotopes in the waste Stream, including any fast neutron poration emitting isotopes alloy with, or disperse in, the chemically Appl. No.: 11/173,271 active metal and the radiation absorbing metals are able to (21) absorb a significant portion of the radioactive emissions (22) Filed: Jul. 1, 2005 asSociated with the isotopes. A transmutation target fraction is included for absorbing fast neutrons and a transmutation Related U.S. Application Data emission absorbing fraction is provided for absorbing emis sions that result from the absorption of a fast neutron by the (63) Continuation of application No. 10/059,808, filed on transmutation target fraction. Non-radioactive constituents Jan. 29, 2002, which is a continuation-in-part of in the waste material are broken down into harmless and application No. 09/334,985, filed on Jun. 17, 1999, useful constituents, leaving the alloyed radioactive isotopes now Pat. -

(A) Two Stable Isotopes of Lithium and Have Respective Abundances of And
NCERT Solution for Class 12 Physics :Chapter 13 Nuclei Q.13.1 (a) Two stable isotopes of lithium and have respective abundances of and . These isotopes have masses and , respectively. Find the atomic mass of lithium. Answer: Mass of the two stable isotopes and their respective abundances are and and and . m=6.940934 u Q. 13.1(b) Boron has two stable isotopes, and . Their respective masses are and , and the atomic mass of boron is 10.811 u. Find the abundances of and . Answer: The atomic mass of boron is 10.811 u Mass of the two stable isotopes are and respectively Let the two isotopes have abundances x% and (100-x)% Aakash Institute Therefore the abundance of is 19.89% and that of is 80.11% Q. 13.2 The three stable isotopes of neon: and have respective abundances of , and . The atomic masses of the three isotopes are respectively. Obtain the average atomic mass of neon. Answer: The atomic masses of the three isotopes are 19.99 u(m 1 ), 20.99 u(m 2 ) and 21.99u(m 3 ) Their respective abundances are 90.51%(p 1 ), 0.27%(p 2 ) and 9.22%(p 3 ) The average atomic mass of neon is 20.1771 u. Q. 13.3 Obtain the binding energy( in MeV ) of a nitrogen nucleus , given m Answer: m n = 1.00866 u m p = 1.00727 u Atomic mass of Nitrogen m= 14.00307 u Mass defect m=7 m n +7 m p - m m=7Aakash 1.00866+7 1.00727 - 14.00307 Institute m=0.10844 Now 1u is equivalent to 931.5 MeV E b =0.10844 931.5 E b =101.01186 MeV Therefore binding energy of a Nitrogen nucleus is 101.01186 MeV.