Instructions for Use Software Version 2.4X © 2021 Welch Allyn
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dissertation (1.448Mb)
Milk revolution and the homogeneous New Zealand coffee market Guo Jingsi 2020 Faculty of Culture and Society A dissertation submitted to Auckland University of Technology in partial fulfilment of the requirements for the degree of Master of Hospitality Supervisor Dr. Lindsay Neill i Abstract It is unsurprising that, as an enjoyable and social beverage, coffee has generated a coffee culture in Aotearoa New Zealand. Part of coffee’s enjoyment and culture is the range of milk types available for milk-based coffees. That range has grown in recent years. A2 Milk is a recent addition to that offering. The A2 Milk Company has experienced exceptional growth. However, my own experience as a coffee consumer in Auckland, Aotearoa New Zealand, has revealed that A2 Milk is not a milk that is commonly offered in many of the city’s cafés. Consequently, my research explores that lack and barista perceptions of A2 Milk within my research at The Coffee Club in Auckland’s Onehunga. As a franchise outlet, The Coffee Club constitutes a representative sample of a wider cohort, the 60 Coffee Clubs spread throughout Aotearoa New Zealand. While my research reinforces much of the knowledge about coffee culture in Aotearoa New Zealand, my emphasis on the influence of A2 Milk within that culture has revealed some interesting new insights. As my five professional barista participants at the Coffee Club revealed, rather than taking a proactive approach to A2 Milk, they were ‘waiting’ for one of two occurrences before considering the offering of A2 Milk. Those considerations included a ‘push’ from the A2 Milk Company that promoted A2 Milk within coffee culture. -

Design Patenting by Organizations, CY 2013
DESIGN PATENTING BY ORGANIZATIONS 2013 March 2014 U.S. PATENT AND TRADEMARK OFFICE ELECTRONIC INFORMATION PRODUCTS DIVISION - PTMT P.O. BOX 1450 ALEXANDRIA, VA 22313-1450 tel (571) 272-5600 / FAX (571) 273-0110 A PATENT TECHNOLOGY MONITORING TEAM REPORT Design Patenting By Organizations 2013 This report, prepared from the Technology Assessment and Forecast (TAF) database, profiles design patents granted during calendar year 2013. Part A1 Part A1 presents patent counts by origin, U.S. and foreign. Individual counts are also presented for each of the top 36 patenting countries. Patent origin is determined by the residence of the first-named inventor listed on a patent. Patent ownership-category information reflects ownership at the time of patent grant and does not reflect subsequent changes in ownership. If more than one assignee (the entity, if any, to which the patent rights have been legally assigned) was declared at the time of grant, a patent is attributed to the ownership-category of the first-named assignee. The "U.S. Corporations" and "Foreign Corporations" ownership categories count predominantly corporate patents; however, patents assigned to other organizations such as small businesses, nonprofit organizations, universities, etc. are also included in these categories. While the "U.S. Government" ownership category includes only patents granted to the Federal Government, no such distinction is made for the "Foreign Government" ownership category. The "U.S. Individuals" and "Foreign Individuals" ownership categories include patents for which ownership was assigned to an individual as well as patents for which no assignment of ownership was made at the time of grant (i.e., ownership was retained by the inventor(s)). -

Bomann KA 167 CB, Glas 8-10 Tassen Braun TA 40Xx Serie
Bomann KA 167 CB, Glas 8-10 Tassen Braun TA 40xx Serie Siemens TK 69009 Siemens TK 52002 Jura Impressa Z7 Alu/Chrom Siemens TK 58001 Philips HD 7544 Philips HD 7686 essential Philips HD 7692 essential Philips HD 7546 / 20 Philips HD 5410 gourmet aluminium Saeco HD 8856/X Exprelia Philips HD 5405 cafe gourmet Saeco HD 5730 one touch Severin KA 5700 Severin KA 4770 Unold 28016 Onyx Unold 28031 White Line Unold 2802x Piano White/Black Unold 28416 Design Serie Saeco HD 8839/X Syntia Cappuccino Saeco Xsmall Steam Saeco Odea Cappucchino Giro Plus E.S. Saeco HD 8944/X Xelsis Saeco SNT 5720 Syntia Cremesso compact manual Bosch TES 50354DE Siemens TK 56004 Cremesso compact automatic Krups EA 8320 Delonghi ECAM 23.420 Delonghi ESAM 6700 Prima Donna Siemens EQ.7 Saeco Royal Cappuccino Saeco Talea Cappuccino xxx Saeco Xsmall Class E.S. Saeco Primea xxx Krups EA 8260 Saeco SLX 6870 Xelsis Krups EA 8250 Jura ENA 9 One Touch Delonghi ESAM 3600 Magnifica Eleg. Pronto Capp. Jura Impressa J7 Krups EA 8245 Bosch TAS 65xx Serie Melitta Caffeo CI Solac CA 4817 Neo Espression Supremma Bosch TCA 6401 Melitta E 960-103 Caffeo Bar Delonghi ESAM 04.110 Magnifica Delonghi ECAM 23.450 Delonghi ESAM 5450 Rapid Cappuccino Delonghi ESAM 3000 Magnifica Delonghi ESAM 5600 Perfecta Delonghi ESAM 5500 Pronto Cappuccino Saeco Talea Ring Plus + Milk Island Gaggia Platinum Swing up Saeco Royal Professional Melitta M 657-xxx Look Therm Melitta M 651-xxx Look Selection Melitta M 652-xxx Look de Luxe Melitta E 950-10x Caffeo Solo Melitta M 650-xxx Look Basis Nespresso Lattissima -
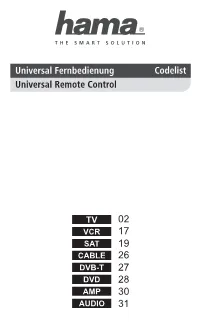
Hama Universal 8-In-1 Remote Control Code List
02 17 19 26 27 28 30 31 TV 01 ACCENT 0301 2551 2791 3601 ANGA 0411 4791 ACCUPHASE 2791 ANGLO 0301 3601 3831 ACEC 2741 ANITECH 0051 0301 1111 2361 ACTION 0051 0301 2091 2391 2391 2551 2581 2791 3811 3601 3831 4731 ADCOM 2711 ANSONIC 5301 5291 0301 1181 ADMIRAL 0051 0301 0621 0741 2551 2581 2631 2741 0981 1131 1571 2571 2791 3601 3881 5581 3661 3831 3881 4641 AOC 0051 2391 4261 ADVENTURA 0411 AR SYSTEM 2551 2791 3321 4761 ADVENTURI 0411 ARC EN CIEL 1981 1991 2091 2341 ADYSON 0051 2141 2391 3911 2591 2641 2681 3921 4731 ARCAM 2141 2641 3911 3921 AEA 2551 2791 4271 AEG 3531 3681 ARCELIK 5591 AGASHI 3831 3911 3921 ARCON 3531 AGB 2141 ARCTIC 5591 AGEF 2571 ARDEM 2551 2791 3541 AIKO 0301 2551 2751 2791 ARISTONA 0051 2471 2551 2741 3601 3831 3891 3911 2791 3921 ARSTIL 5591 AIM 2551 2791 3681 3721 ART TECH 0051 3841 ARTHUR-MARTIN 0621 0771 3881 AKAI 5301 5291 0051 0161 ASA 1101 1371 1381 1471 0301 0411 0561 0671 2571 2951 3881 4631 0741 0871 0991 1001 4641 ASBERG 0051 1111 1571 2551 1271 1291 1491 2141 2581 2791 2321 2361 2371 2391 ASORA 0301 3601 2551 2751 2791 3331 ASTON 4281 3541 3601 3681 3711 ASTRA 0301 2551 2791 3721 3831 3881 3891 ASTRELL 5081 3901 3911 3921 4791 5581 ASUKA 2141 2361 3831 3911 3921 AKASHI 3601 ATD 3841 AKIBA 2361 2551 2791 ATLANTIC 0051 1901 2551 2791 AKITO 2551 2791 3111 3911 AKURA 0051 0301 0881 0891 ATORI 0301 3601 2361 2551 2791 3541 ATORO 0301 3601 3831 AUCHAN 0621 0771 3881 ALARON 3911 AUDIOSONIC 0051 0301 1181 2091 ALBA 5301 5291 0051 0301 2141 2361 2551 2591 1181 1571 1681 2141 2631 2791 3331 3541 2361 2371 2491 -

Home Comforts Chip Maintenance
CHIP MAINTENANCE FEE CATEGORY DESCIPTION PRICE REWARD POINTS Classic ANNUAL CHIP MAINTENANCE FEE 2200 5,500 Gold ANNUAL CHIP MAINTENANCE FEE 3400 8,500 Platinum ANNUAL CHIP MAINTENANCE FEE 6800 17,000 HOME COMFORTS EASY SHOP CATEGORY ITEM CODE BRAND DESCIPTION PURCHASE PRICE I-SHOP On Card On Reward Points Home Appliances HK-101 MOULINEX HAND BLENDER 4,658 11,644 2,329 5,822 Home Appliances HK-102 MOULINEX SANDWICH MAKER 3,220 8,050 1,610 4,025 Home Appliances HK-103 MOULINEX KETTLE 1.7 LTR 5,520 13,800 2,760 6,900 Home Appliances HK-104 MOULINEX COFFEE MAKER 4-6 Cups 4,485 11,213 2,243 5,606 Home Appliances HK-105 MOULINEX CHOPPER + MEAT MINCER 5,376 13,441 2,688 6,720 Home Appliances HK-106 MOULINEX 2 SLICE TOASTER 5,290 13,225 2,645 6,613 Home Appliances HK-107 MOULINEX BLENDER GRINDER 8,280 20,700 4,140 10,350 Home Appliances HK-108 MOULINEX FOOD PROCESSOR 19,320 48,300 9,660 24,150 Home Appliances HK-109 MOULINEX DEEP FRYER 8,280 20,700 4,140 10,350 Home Appliances HK-110 MOULINEX VACUUM CLEANER 5,635 14,088 2,818 7,044 Home Appliances HK-111 MOULINEX FRUIT JUICER 12,869 32,171 6,434 16,086 Home Appliances HK-112 MOULINEX CITRUS JUICER 3,048 7,619 1,524 3,809 Home Appliances HK-113 TEFAL SANDWICH MAKER 4,140 10,350 2,070 5,175 Home Appliances HK-114 TEFAL MEAT GRILL 15,525 38,813 7,763 19,406 Home Appliances HK-115 TEFAL FOOD STEAMER 11,960 29,900 5,980 14,950 Home Appliances HK-116 TEFAL GARMENT STEAMER 8,385 20,963 4,193 10,481 Home Appliances HK-117 TEFAL TOASTER 6,210 15,525 3,105 7,763 Home Appliances HK-118 TEFAL DRY IRON 3,335 -

Han Van Splunter Updated
DAP Han van Splunter CEO DAP November 23, 2004 Mission statement " DAP offers consumers exciting experiences that help them look, feel and live better, thanks to breakthrough products that combine advanced technology with a deep understanding of what consumers seek with respect to health, beauty and home care" DAP 2 Agenda • DAP overview • Sustained financial performance – Continuing profitable growth – Enhancing operational performance • Conclusion DAP 3 Divisional Strategy • Consolidating and achieving leadership positions in main categories through superior value propositions for retailers and end-users • Breakthrough concepts to accelerate growth and to change the dynamics in mature markets • Further improvements in operational excellence, e.g. through asset management and diversity reduction DAP 4 DAP - setting the scene In 2004, DAP is selling 77 million products to consumers worldwide DAP 5 Overview of businesses • Personal Care – Shaving & Beauty – Oral Health Care – Health & Wellness • Food & Beverage • Home Environment Care DAP 6 Sales by Global Business Unit YTD September 04 Shaving & Beauty has the largest share of the DAP sales 20% 23% 44% 13% Shaving & Beauty Oral Healthcare Food & Beverage Home Environment Care DAP 7 Sales by Region YTD September 04 EMEA Region represents the majority of the DAP sales 18% 5% 20% 57% EMEA North America Latin America Asia Pacific DAP 8 DAP shows high operational margins €M 450 20% 400 18% 350 16% 300 14% 12% 250 10% 200 8% 150 6% 100 4% 50 2% 0 0% 1999 2000 2001 2002 2003 IFO IFO as % of sales -

Supplier List
Current as of October 2020 Supplier List 3M Company ASP Global CareFusion 213, LLC Abbott Laboratories, Inc. Aspen Surgical Products, Inc. CareFusion 2200, Inc. Abbott Nutrition AuroMedics Pharma LLC CareFusion 303, Inc. ABM Healthcare Avadim Technologies Inc Carestream Health Inc Accord Healthcare Inc Avanos Medical, Inc. CDW Accountable Healthcare Staffing, Inc. Axion Contact Center, LLC Century Linen & Uniform ACell Inc. Aztec Office Solutions Cepheid Action Health B. Braun Medical Inc. Chem-Aqua Activation Systems, LLC Bake'n Joy Chiesi USA Inc. Advance Medical Designs, Inc. Bard Access System Cianna Medical, Inc Advanced Medical Inc. d/b/a Vygon USA Bard Medical Division Cimplx HR, Inc. AdvoWaste Medical Services, LLC Bard Peripheral Vascular Inc Cintas Corporation Afflink, Inc. Baxter Healthcare Corporation Bioscience Clean Harbors Environmental Services Inc Agfa US Corp. Baxter Healthcare Corporation Medical ClearEdge Partners, Inc. Airgas, Inc. Products - Specialty Pharma ClearSpec Medical, LLC Alco Sales & Service Co Bayer Healthcare Clinical Innovations, Inc. Alimed Beaver Visitec International Inc Clorox Sales Company All Pro Cleaning Systems Beckman Coulter Inc Coloplast Corp. Allergan USA Inc Becton Dickinson and Company Comprehensive Pharmacy Services, LLC Allseating Corporation Bimbo Bakeries USA, Inc. (aka CPS) Ambu, Inc. bioMerieux, Inc. Conmed Corporation Amd-Ritmed Inc. Bio-Rad Laboratories Conmed/Linvatec American Food and Vending Biotronik, Inc. ConsortiEx American Regent Laboratories, Inc. Blue Ridge X-Ray Co Inc Contec American Solutions For Business Boss Instruments, Ltd. ConvaTec Inc Amneal Pharmaceuticals, LLC Boston Scientific Company Cook Medical LLC Amsino International, Inc. Boston Textile Corporation Copier Audit LLC DBA Copier Analytics Andover Healthcare Inc Bracco Diagnostics, Inc. Corflex Inc. Angiodynamics Inc Breg, Inc Costa Fruit & Produce Ansell Healthcare Products Inc BSN Medical, Inc. -

Longevity and Sharing Expertise
Volume 2 n 2017 | The Magazine for Partners of B. Braun’s OEM Division THE EXPERIENCE OF QUALITY: LONGEVITY AND SHARING EXPERTISE THE STATE OF THE MEDICAL DEVICE INDUSTRY: Q & A WITH THE MDMA’S MARK LEAHEY CONTENTS 4-5 6 8-11 Editor’s Note Ask the Expert: The Experience of Bridseida Melendez Quality: Longevity and Sharing Expertise 12-13 14-17 18-19 New NRFit™ Design Skill and Quality in Our Through the Lens: Helps Eliminate Hands – Manufacturing Needle-Free Manufacturing Improper Connections Excellence in the Dominican Republic 20-21 22-23 24-27 Meet the OEM & n Educating While Entertaining Show Time: Making International Team n New Faces at B. Braun OEM the Most of Your n What Is This? Trade Show Visit CHECK OUT OUR MEDICAL CRYPTOGRAM PUZZLE ON PAGE 43! | 28-32 Volume 2 2017 Publisher: B. Braun Medical Inc. Editor-in-Chief: Tom Black Contributing Editor: Dave Williams Co-Contributing Editors: The State of the Medical Allison Longenhagen, Chad Laity Device Industry: An Interview with the Design & Production: Lehigh Mining & Navigation MDMA’s Mark Leahey Creative Director: Denis Aumiller Design: Katie Sak Project Manager: Cathy Wagner 34-35 Copywriter: Michael Drabenstott Production Assistant: Pat Lundy Printing: Doug Earp Photography: John Sterling Ruth Studio “What I’ve Learned” B. Braun OEM USA – bboemchannel.com linkedin.com/company/b--braun-medical B. BRAUN MEDICAL INC. 824 Twelfth Avenue 36-41 Bethlehem, Pa. 18018 TF. 1.800.523.9676 P. 610.691.5400 us.bbraunoem.com © 2017 B. Braun Medical Inc., Bethlehem, Pa. All rights reserved. OEM 17-5922 5/1 LMN How B. -

Pushing Regional Studies Beyond Its Borders? Spatial Economic Analysis Lecture Territory, Politics, Governance Annual Lecture
REGIONAL STUDIES ASSOCIATION ANNUAL CONFERENCE @regstud PUSHING REGIONS BEYOND THEIR BORDERS #RSASdC UNIVERSITY OF SANTIAGO DE COMPOSTELA, SPAIN 4TH – 7TH JUNE SME At a Glance Conference Programme Tuesday 4th June 2019 10.00-16.00 RSA Board Meeting 15.00-17.00 City Walking Tour 17.00-18.00 Conference Registration Wednesday 5th June 2019 08.00-17.30 Conference registration 09.00-09.30 Welcome to the conference 09.30-11.00 Opening Plenary Panel Session organised by Urban and Regional Horizons section of Regional Studies Pushing Regional Studies beyond its Borders? Chair: John Harrison, Loughborough University, UK Panelists • Mercedes Delgado, MIT, USA • Ben Derudder, Ghent University, Belgium • Isabelle Anguelovski, Universitat Autònoma de Barcelona, Spain • Sergio Montero Muñoz, Universidad de los Andes, Colombia • David Bailey, Aston University, UK 11.00-11.30 Coffee Break 11.30-13.00 Parallel Workshop Sessions 1 13.00-14.30 Lunch Break 13.00-14.30 Territorial Representatives annual meeting and lunch (Invitation only) 14.30-15.15 Spatial Economic Analysis Lecture Chair: Paul Elhorst, University of Groningen, The Netherlands Speaker: Rachel Franklin, CURDS, Newcastle University, UK 15.15-16.45 Parallel Workshop Sessions 2 16.45-17.15 Coffee Break 17.15-18.00 Territory, Politics, Governance Annual Lecture Chair: Klaus Dodds, Royal Holloway University of London, UK Speaker: John Agnew, UCLA, USA 18.15-20.30 Welcome Reception - Coaches will leave the conference venue between 18:15-18:30 1 REGIONAL STUDIES ASSOCIATION ANNUAL CONFERENCE @regstud -

A Global Ambition a Thriving Medtech Economy a Global Ambition a Thriving Medtech Economy
MEDICAL TECHNOLOGY INDUSTRY SECTOR BLUEPRINT 2011 A global ambition A thriving medtech economy A global ambition A thriving medtech economy The Medical Technology Association of New Zealand (MTANZ) is the leading industry body representing medical technology manufacturers, importers and distributors of medical devices in New Zealand. MTANZ aims to increase awareness of the medical technology industry in New Zealand and to ensure patients benefit from the innovative medical devices available today. MTANZ supports New Zealand researchers and manufacturers in developing medical devices for international markets. MTANZ represents manufacturers and suppliers of medical technology used in the diagnosis, prevention, treatment and management of disease and disability. MTANZ promotes policies for a legal, regulatory and economic environment to advance access to innovative medical technologies. THE MEDICAL TECHNOLOGY ASSOCIATION OF NEW ZEALAND PO Box 74116, Greenlane Central, Auckland 1546 P + 64 9 917 3645 F + 64 9 917 3651 2 3 A global ambition A thriving medtech economy The medical Over the next 24 technology sector of months, 22 companies New Zealand in the New Zealand plans to hire over medical technology 165 full-time sector plan to raise skilled workers $44.1 million. in the next 24 months. In the last financial The New Zealand year, the New Zealand medical technology medical technology sector spent over sector earned over $65.3 million on $578.8 million in research and exports. development in the last financial year. www.mtanz.org.nz 2 3 A global ambition A global ambition A thriving medtech economy A thriving medtech economy ACKNOWLEDGEMENTS EXECUTIVE SUMMARY 1. THE ROLE OF MEDICAL TECHNOLOGY IN NEW ZEALANd’s ECONOMY 1.1 National Prosperity and Medical Devices 1.2 Increasing Our Share of the TIN100 1.3 Closing the Gap with Australia 1.4 Building a “Healthy” Healthcare System 1.5 Incubating Growth 1.6 What Success Looks Like for Medical Technology 2. -

Schlüter®-BEKOTEC-THERM Ceramic Thermal Comfort Floor
Schlüter®-BEKOTEC-THERM Ceramic thermal comfort floor Technical Manual Schlüter®-BEKOTEC-THERM Werner Schlüter SCHLÜTER-SYSTEMS KG 2 Schlüter®-BEKOTEC-THERM About this manual: The design principle of the ceramic thermal comfort floor The innovative Schlüter®-BEKOTEC-THERM heating system is referred to as a “ceramic ther- mal comfort floor,” to emphasise the fact that our company views the heating system of the floor as a system assembly, in which all components, design planning, and construction must seam- lessly fit together. After all, to meet the many requirements, the “ceramic thermal comfort floor” must provide insulation, heating, cooling and waterproofing in wet areas, while also absorbing traffic loads and serving as a visually appealing design element. The experience of the past has shown how difficult it is to achieve a satisfactory balance between the aspects of construction, physics and heating technology in the overall floor assembly. As a consequence, conventional heated screeds with ceramic and natural stone coverings frequently Reducing stresses in the screed... buckle and form cracks. This is mainly attributable to the fact that screed and ceramics expand and contract at different rates due to their different heat expansion coefficients during temperature changes. The provisions of the corresponding standards, for example the regulations that specify the thick- ness of the screed, the position and type of movement joints, the construction of reinforcement inserts, or the maximum residual moisture for tile installation, do not solve the physical problems encountered in construction. From the perspective of heating technology, the disadvantage of a relatively large mass of screed is that a great amount of heating energy must initially be used and stored. -

Small Appliances Market - Global Industry Trends, Analysis, Forecasts 2018
Jul 11, 2016 04:25 EDT Small Appliances Market - Global Industry Trends, Analysis, Forecasts 2018 Small appliances market is forecasted to grow substantially in all market segments through 2016 owing to the rise in living standards and need for more comfort. Small appliance industry consists of home appliances that are movable or partially movable and can be used on tables, counters, or other platforms. The growth of small appliances market is expected to be driven by product innovation, upgradation of existing products, and value-added features in the coming years. Small appliances are generally categorized as commercial and home appliances. Commercial appliances are meant for business or office settings, while home appliances are used for household purposes. Popular home appliances target health issues and promise consumer restaurant experience at home. • Some of the major advantages of using these small appliances are: Time saving Reduction in manual efforts Effective results Market Segmentation • Based on Products Food Preparation Appliances Heating Appliances Irons Personal Care Appliances Small Cooking Appliances Small Kitchen Appliances (Non-cooking) Vacuum Cleaners • Get Free Sample Report Copy This research report provides an analysis of the market depending on its major market segments and geographies.The major regions analyzed under this research report are: • Europe • North America • Asia-Pacific • Rest of the World This report provides a complete analysis of the major industry segments, current market trends, market structure, industry growth drivers, restraints, and market projections for the coming years. It also includes analysis of technological developments in the small appliances market, Porter’s five force model analysis, and detailed company profiles of the top industry players.