List of Participants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -
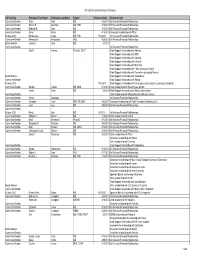
CB2013 Committee and Board Disclosureauditlist 9.10.13
2013-2014 Conflict of Interest Disclosure AST Activity Participant First Name Participant Last Name Degree DisclosureDate Disclsoure Item Committee Member Reza Abdi MD 4/22/2013 No Relevant Financial Relationships Committee Member Sameh R. Abul-Ezz MD, PhD 4/16/2013 No Relevant Financial Relationships Committee Member Deborah B. Adey MD 4/15/2013 No Relevant Financial Relationships Committee Member Enver Akalin MD 4/16/2013 Consultant relationship with Pfizer Fellows 2013 Maria-Luisa Alegre MD, PhD 7/12/2013 No Relevant Financial Relationships Committee Member Alessandrini Alessandro PhD 4/29/2013 No Relevant Financial Relationships Board Member James S. Allan MD 5/3/2013 Committee Member No Relevant Financial Relationships Rita R. Alloway PharmD, FCCP Grant Support relationship with Astellas Grant Support relationship with BMS Grant Support relationship with Novartis Grant Support relationship with Lifecycle Grant Support relationship with Millenium Grant Support relationship with Pfizer (previously Wyeth) Grant Support relationship with Genentech (previously Roche) Board Member Grant Support relationship with Viropharma Committee Member Grant Support relationship with Alexion Fellows 2013 7/18/2013 Grant Support relationship with Sanofi (previously Genzyme, previously Sangstat) Committee Member Sandra Amaral MD, MHS 6/18/2013 Other relationship with Bristol Myers-Squibb Hatem Amer MD 5/6/2013 Grant Support relationship with Abbott Laborartories. Committee Member Other relationship with Massacheussetts Medical Society. Committee Member William Applegate No Relevant Financial Relationships Committee Member Alexander Aussi BSN, RN, MBA 4/22/2013 Consultant relationship with Total Transplant Advantage, LLC Committee Member Jamil Azzi MD 4/30/2013 No Relevant Financial Relationships Committee Member Fellows 2013 Mark L. -

AMGEN INC. V. SANOFI
Case: 20-1074 Document: 159 Page: 1 Filed: 06/21/2021 NOTE: This order is nonprecedential. United States Court of Appeals for the Federal Circuit ______________________ AMGEN INC., AMGEN MANUFACTURING, LIMITED, AMGEN USA, INC., Plaintiffs-Appellants v. SANOFI, AVENTISUB LLC, FKA AVENTIS PHARMACEUTICALS INC., REGENERON PHARMACEUTICALS INC., SANOFI-AVENTIS U.S. LLC, Defendants-Appellees ______________________ 2020-1074 ______________________ Appeal from the United States District Court for the District of Delaware in Nos. 1:14-cv-01317-RGA, 1:14-cv- 01349-RGA, 1:14-cv-01393-RGA, 1:14-cv-01414-RGA, Judge Richard G. Andrews. ______________________ JEFFREY A. LAMKEN, MoloLamken LLP, Washington, DC, filed a petition for rehearing en banc for plaintiffs-ap- pellants. Also represented by SARAH JUSTINE NEWMAN, MICHAEL GREGORY PATTILLO, JR.; SARA MARGOLIS, New York, NY; EMILY JOHNSON, ERICA S. OLSON, STEVEN TANG, STUART WATT, WENDY A. WHITEFORD, Amgen Inc., Thou- sand Oaks, CA; KEITH HUMMEL, Cravath Swaine & Moore LLP, New York, NY; WILLIAM G. GAEDE, III, McDermott Case: 20-1074 Document: 159 Page: 2 Filed: 06/21/2021 2 AMGEN INC. v. SANOFI Will & Emery LLP, Menlo Park, CA; CHRISTOPHER B. MEAD, Schertler Onorato Mead & Sears LLP, Washington, DC; JAMES L. HIGGINS, MELANIE K. SHARP, Young, Cona- way, Stargatt & Taylor, LLP, Wilmington, DE. Plaintiff- appellant Amgen Inc. also represented by SARAH CHAPIN COLUMBIA, McDermott, Will & Emery LLP, Boston, MA; LAUREN MARTIN, Quinn Emanuel Urquhart & Sullivan LLP, Boston, MA. MATTHEW WOLF, Arnold & Porter Kaye Scholer LLP, Washington, DC, filed a response for defendants-appellees. Also represented by VICTORIA REINES; DAVID K. BARR, DANIEL REISNER, New York, NY; DEBORAH E. -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

ALEXION PHARMACEUTICALS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K Current Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): May 4, 2021 ALEXION PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) Delaware 000-27756 13-3648318 (State or other jurisdiction of incorporation) (Commission File Number) (IRS Employer Identification No.) 121 Seaport Boulevard, Boston, Massachusetts 02210 (Address of principal executive offices, including zip code) (475) 230-2596 (Registrant’s telephone number, including area code) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: ☒ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: Trading Name of each exchange Title of each class Symbol on which registered Common Stock, par value $0.0001 per share ALXN The Nasdaq Global Select Market Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). -

1976 3M Medical Solutions Division 2019 Electrocore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc
CURRENT SUSTAINING MEMBER COMPANIES MEMBER FOR OVER: 10 Years 25 Years 50 Years Member Since (alphabetical order) 1976 3M Medical Solutions Division 2019 electroCore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc. 2010 Endo Pharmaceuticals 2018 Organogenesis 2013 AbbVie Inc. 2017 Exelixis 2004 Otsuka America Pharmaceutical, Inc. 2021 Adaptive Biotechnologies 2016 Express Scripts Federal Pharmacy 2018 Pacira BioSciences, Inc. 2017 ACADIA Pharmaceuticals, Inc. 2010 Federal Practitioner 2018 Paratek Pharmaceuticals 2020 AcelRx Pharmaceuticals, Inc. 2018 Foundation Medicine, Inc. 1990 Pfizer Pharmaceuticals 2020 Acorda Therapeutics 2021 Frontier Technology Inc. (FTI) 2017 Pharmacyclics, LLC 2019 Aimmune 2020 Fresenius Medical Care North America 2020 RedHill BioPharma 2003 Alcon Laboratories, Inc. 1989 Genentech Inc. 2019 Red One Medical 2019 Alexion Pharmaceuticals, Inc. 2006 Gilead Sciences 2020 Regeneron 2017 Alkermes, Inc. 1983 GLAXOSMITHKLINE 2009 Regenesis Biomedical, Inc. 2019 Alnylam Pharmaceuticals 2013 Golden State Medical Supply, Inc. 2011 Remund Group, LLC 2019 Altarum Institute 2020 GRAIL 2018 Rigel Pharmaceuticals 2020 Amarin Corporation 2019 Greenwich Biosciences 2000 Sanofi 1994 AmerisourceBergen 2013 Gulf Coast Pharmaceuticals Plus, LLC 2020 Seattle Genetics 1992 Amgen 2008 Heritage Health Solutions, Inc. 2004 Siemens Medical Solutions 2020 Amneal Pharmaceutical 2017 Hill-Rom Company 2019 SK Life Science, Inc. 2019 Aptive Resources LLC 2020 Immunomedics 2002 Smith & Nephew, Inc. 2020 The Arbinger Institute 2019 ImmunoVation, LLC 2019 Sobi Inc. 2011 Arbor Pharmaceuticals, LLC 2019 Incyte Corporation 2013 Stryker Orthopaedics 2010 Argentum Medical, LLC 2019 Indivior 2018 Sun Pharmaceutical 2019 ASM Research, LLC 2015 Intercept Pharmaceuticals 1999 Sunovion Pharmaceuticals, Inc. 1986 Astellas Pharma US, Inc. 2019 Ipsen Biopharmaceuticals, Inc. 2016 Taiho Oncology, Inc. 1995 AstraZeneca 2018 IT Cadre 2015 Takeda Oncology 2020 Baudax Bio, Inc. -

Federal Register/Vol. 84, No. 232/Tuesday, December 3, 2019
Federal Register / Vol. 84, No. 232 / Tuesday, December 3, 2019 / Notices 66191 the Assistant Attorney General, patterns, devices, manufacturing filed with and accepted, subject to final developed the HSR Rules and the processes, or customer names. approval, by the Commission, has been corresponding Notification and Report placed on the public record for a period Form. Heather Hippsley, of thirty (30) days. The following On September 11, 2019, the Deputy General Counsel. Analysis to Aid Public Comment Commission sought comment on the [FR Doc. 2019–26075 Filed 12–2–19; 8:45 am] describes the terms of the consent reporting requirements associated with BILLING CODE 6750–01–P agreement and the allegations in the the HSR Rules and corresponding complaint. An electronic copy of the Notification and Report Form. 84 FR full text of the consent agreement 47951. No relevant comments were FEDERAL TRADE COMMISSION package can be obtained from the FTC received. Pursuant to the OMB [File No. 191 0061] Home Page (for November 15, 2019), on regulations, 5 CFR part 1320, that the World Wide Web, at https:// implement the PRA, 44 U.S.C. 3501 et Bristol-Myers Squibb Company and www.ftc.gov/news-events/commission- seq., the FTC is providing this second Celgene Corporation; Analysis of actions. opportunity for public comment while Agreement Containing Consent Orders You can file a comment online or on seeking OMB approval to renew the pre- To Aid Public Comment paper. For the Commission to consider existing clearance for those information your comment, we must receive it on or collection requirements. -

Shire Acquisition of Viropharma - Strategic Move to Strengthen Shire’S Rare Disease Business - Augments Already Strong Growth Prospects
Shire acquisition of ViroPharma - Strategic move to strengthen Shire’s Rare Disease business - Augments already strong growth prospects Flemming Ornskov, MD Chief Executive Officer Graham Hetherington Chief Financial Officer Our purpose We enable people with life-altering conditions to lead better lives. CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS Statements included in this communication that are not historical facts are forward-looking statements. Forward-looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: •Shire’s proposed acquisition of ViroPharma may not be consummated due to the occurrence of an event, change or other circumstances that gives rise to the termination of the merger agreement; •a governmental or regulatory approval required for the proposed acquisition of ViroPharma may not be obtained, or may be obtained subject to conditions that are not anticipated, or another condition to the closing of the proposed acquisition may not be satisfied; •ViroPharma may be unable to retain and hire key personnel and/or maintain its relationships with customers, suppliers and other business partners pending the consummation of the proposed acquisition by Shire, or ViroPharma’s business may be disrupted by the proposed acquisition, including increased costs and diversion of management time and resources; •difficulties in integrating ViroPharma into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; and risks and uncertainties detailed from time to time in Shire’s or ViroPharma’s filings with the U.S. -

Pharma: Strategic Realignment for a 112/113 Better Future Prism / 2 / 2020
Pharma: Strategic realignment for a 112/113 better future Prism / 2 / 2020 Pharma: Strategic realignment for a better future ... and how the industry will be forced to overcome its hesitation to innovate in operations Ben van der Schaaf, Aurelien Guichard The life sciences sector faces significant impact from Amid the search for COVID-19 – and although the race for treatments and vaccines effective COVID-19 treatments and vaccines, is dominating the headlines, the effect on the industry will the pandemic will have not solely be positive. long-term side effects for the global pharmaceutical In the short term, some companies industry. As our article explains, companies are laying people off and reducing will need to focus on operations, whereas others are change in three areas reallocating resources to focus on (portfolio reprioritization, COVID-19, or even ramping up accelerated R&D and technology efforts in other areas. Stock-market transformation) if they performance has been as diverse are to position (Figure 1). themselves successfully for the future. Organizations need to consider major strategic questions now, to ensure their success in the longer term. The industry is clearly in the middle of the efforts to combat COVID-19: - More than 20 companies are trying to find a treatment with either new or approved drugs1. - More than 15 companies globally are mobilizing resources to develop new vaccines2. - Globally, by the end of May, more than 1,300 clinical trials related to COVID-19 were recruiting patients3. 1. Marketwatch.com, 6 May 2020 2. Drugtargetreview.com, 9 April 2020 3. clintrials.gov, 31 May 2020 Pharma: Strategic realignment for a 114/115 better future Prism / 2 / 2020 2. -

Package Insert Has Been Approved by the U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION **Doses up to 1,000 U every 3 to 4 days may be considered based on These highlights do not include all the information needed to use individual patient response. ® CINRYZE safely and effectively. See full prescribing information for ---------------------- DOSAGE FORMS AND STRENGTHS -------------------- CINRYZE. Approximately 500 Units (lyophilized) in an 8 mL vial. (3) CINRYZE (C1 Esterase Inhibitor [Human]) ---------------------------- CONTRAINDICATIONS ------------------------------- For Intravenous Use, Freeze-Dried Powder for Reconstitution Patients who have manifested life-threatening immediate hypersensitivity Initial U.S. Approval: 2008. reactions, including anaphylaxis, to the product (4). --------------------------- RECENT MAJOR CHANGES -------------------------- ----------------------------- WARNINGS/PRECAUTIONS ------------------------ • Indications and Usage (1) 06/2018 • Hypersensitivity reactions may occur. Have epinephrine immediately • Dosage and Administration (2.1) 06/2018 available for treatment of acute severe hypersensitivity reaction (5.1) • --------------------------- INDICATIONS AND USAGE --------------------------- Serious arterial and venous thromboembolic (TE) events have been CINRYZE is a C1 esterase inhibitor indicated for routine prophylaxis against reported at the recommended dose of C1 Esterase Inhibitor (Human) angioedema attacks in adults, adolescents and pediatric patients (6 years of products, including CINRYZE, following administration in patients with age and older) -

BIOGEN INTERNATIONAL GMBH, Plaintiff-Appellant
Case: 20-1373 Document: 41 Page: 1 Filed: 04/21/2020 United States Court of Appeals for the Federal Circuit ______________________ BIOGEN INTERNATIONAL GMBH, Plaintiff-Appellant v. BANNER LIFE SCIENCES LLC, Defendant-Appellee ______________________ 2020-1373 ______________________ Appeal from the United States District Court for the District of Delaware in No. 1:18-cv-02054-LPS, Chief Judge Leonard P. Stark. ______________________ Decided: April 21, 2020 ______________________ JAMES B. MONROE, Finnegan, Henderson, Farabow, Garrett & Dunner, LLP, Washington, DC, for plaintiff-ap- pellant. Also represented by PAUL WILLIAM BROWNING, J. MICHAEL JAKES, LAURA POLLARD MASUROVSKY, JASON LEE ROMRELL. KYLE MUSGROVE, Parker Poe Adams & Bernstein LLP, Charlotte, NC, for defendant-appellee. Also represented by JOHN WORTHINGTON BATEMAN, ELIZABETH CROMPTON, SCOTT A. CUNNING, II, Washington, DC. ______________________ Case: 20-1373 Document: 41 Page: 2 Filed: 04/21/2020 2 BIOGEN INTERNATIONAL GMBH v. BANNER LIFE SCIENCES LLC Before LOURIE, MOORE, and CHEN, Circuit Judges. LOURIE, Circuit Judge. Biogen International GmbH (“Biogen”) appeals from a judgment of the United States District Court for the Dis- trict of Delaware that Banner Life Sciences LLC (“Banner”) does not infringe the extended portion of U.S. Patent 7,619,001 (the “’001 patent”), extended under the patent term restoration provisions of the Hatch-Waxman Act, Pub. L. No. 98-417, § 201, 98 Stat. 1585, 1598 (as codified at 35 U.S.C. § 156 (2018)). Biogen Int’l GmbH v. Banner Life Scis. LLC, No. 18-2054-LPS, 2020 WL 109499 (D. Del. Jan. 7, 2020) (“Decision”). Because the scope of a patent term extension under 35 U.S.C. -

Human Sequencing Resource with UK Biobank
January 8, 2018 Regeneron Forms Consortium of Leading Life Sciences Companies to Accelerate Largest Widely-Available 'Big Data' Human Sequencing Resource with UK Biobank By end of 2019, Regeneron plans to sequence the exomes of all 500,000 people within the UK Biobank resource, all with associated health records, creating an unprecedented resource linking human genetic variations to human biology and disease AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen and Pfizer will join Regeneron to co-fund one of the industry's most ambitious 'pre-competitive' research efforts Exome sequencing data and findings will be openly available to other researchers TARRYTOWN, N.Y., Jan. 8, 2018 /PRNewswire/ -- Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN), along with new collaborators AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen and Pfizer Inc., today announced the formation of a major 'pre-competitive' consortium to fund the generation of genetic exome sequence data from the 500,000 volunteer participants who make up the UK Biobank health resource. The newly announced collaborators will each commit $10 million to enable a dramatic acceleration of sequencing timelines, and additional companies are considering joining the consortium. Regeneron will conduct the sequencing effort. The sequencing data will be paired with detailed, de-identified medical and health records within the UK Biobank resource, including enhanced measures such as brain, heart and body imaging, to create an unparalleled resource for linking human genetic variations to human biology and disease. It was originally planned that sequencing of all 500,000 samples in the UK Biobank would be completed by 2022, with the first 50,000 people sequenced during 2017 with funding from Regeneron and GlaxoSmithKline.