Identifying Button Cell Batteries That Contain Mercury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Varta Msds Primary Zinc Air B
Safety Data Sheet MSDS 2.001.019 Primary zinc/air button cell, mercury free (Series: p...MF) 1 Identification of the product and of the company undertaking Product details Trade name Primary zinc/air button cell Voltage 1.4 V Electrochemical system: Zinc / KOH electrolyte / Oxygen Anode (negative electrode): Zinc Cathode (positive electrode): MnOx catalyst This MSDS applies to the following cell types and all varieties supplied by VARTA Microbattery. Type IEC battery designation ANSI battery designation p 675 MF IEC PR 44 ANSI 7003ZD p 13 MF IEC PR 48 ANSI 7000ZD p 312 MF IEC PR 41 ANSI 7002ZD p 10 MF IEC PR 70 ANSI 7005ZD p 675 IMP MF IEC PR 44 9V block MF ANSI 7004Z Supplier details Address: VARTA Microbattery GmbH Daimlerstraße 1 D-73479 Ellwangen Germany Emergency Phone Number: +49 7961 921 110 (VAC) General remark This information is provided as a service to our customers. The details presented are in accordance with our present knowledge and experiences. They are no contractual assurances of product attributes. Legal remark (EU) These batteries are no “substances” or “mixtures” according to Regulation (EC) No 1907/2006 EC. Instead they have to be regarded as “articles”, no substances are intended to be released during handling. Therefore there is no obligation to supply a safety data sheet according to Regulation (EC) 1907/2006, Article 31. Legal remark (USA) Safety Data Sheets are a sub-requirement of the Occupational Safety and Health Administration (OSHA) Hazard Communication Standard, 29 CFR Subpart 1910.1200. This Hazard Communication Standard does not apply to various subcategories including Page no.: 1 of 8 VARTA Microbattery GmbH Edition: 14.05.2019 Electronically generated document - no signature required. -

Hydrogel Leclanché Cell: Construction and Characterization
energies Article Hydrogel Leclanché Cell: Construction and Characterization Greg Jenson 1,2,* , Gurjap Singh 2,3 , Jay K. Bhama 2,4,5 and Albert Ratner 2,3 1 Department of Surgery, University of Iowa Hospitals and Clinics, Iowa City, IA 52242, USA 2 Bhama-Ratner Artificial Heart & MCS Advancement Lab, University of Iowa Department of Mechanical Engineering, 3131 Seamans Ctr, Iowa City, IA 52242, USA; [email protected] (G.S.); [email protected] (J.K.B.); [email protected] (A.R.) 3 Department of Mechanical Engineering, University of Iowa, Iowa City, IA 52242, USA 4 Baptist Health Medical Center, Little Rock, AR 72205, USA 5 Division of Cardiovascular Surgery, University of Arkansas for Medical Sciences, University of Arkansas for Medical Sciences, 4301 W Markham, Little Rock, AR 72205, USA * Correspondence: [email protected] Received: 10 December 2019; Accepted: 21 January 2020; Published: 28 January 2020 Abstract: A liquid-to-gel based Leclanché cell has been designed, constructed and characterized for use in implantable medical devices and other applications where battery access is limited. This well-established chemistry will provide reliable electrochemical potential over a wide range of applications and the novel construction provides a solution for the re-charging of electrodes in hard to access areas such as an internal pacemaker. The traditional Leclanché cell, comprised of zinc (anode) and manganese dioxide (cathode), conductive carbon powder (acetylene black or graphite), and aqueous electrolyte (NH4Cl and ZnCl2), has been suspended in an agar hydrogel to simplify construction while maintaining electrochemical performance. Agar hydrogel, saturated with electrolyte, serves as the cell support and separator allowing for the discharged battery suspension to be easily replaced once exhausted. -

Batteries Information Received from EU, Canada, Japan, Indonesia, USA and Other Stakeholders (BAJ, IPEN, NRDC, ZMWG)
Batteries Information received from EU, Canada, Japan, Indonesia, USA and other stakeholders (BAJ, IPEN, NRDC, ZMWG) 1. Category of mercury-added product Batteries 2. Further description of the product Mercury-containing button cells 3. Information on the use of the Currently, there are three types of button cell batteries that contain mercury: zinc air, silver oxide and alkaline. product These batteries contain mercury in small amounts (typically 0.1-2%) and the purpose of mercury in the cell is to prevent the build-up of hydrogen gas. The mercury acts as a barrier to the production of hydrogen and as such prevents the cell swelling and becoming damaged. Figure 1 – Cross Section of Zinc Anode Button Cell and Zinc Air Button Cell (European Commission, 2014) Range of mercury content/consumption per unit product - 0.1 – 2 weight-% (button cells with intentionally added mercury) - 0.0005 weight-% (button cells without intentionally added mercury) Button batteries are used for powering high drain devices such as watches, calculators, and hearing aids. 4. Information on the availability of EU mercury-free (or less-mercury) Main alternatives: Mercury-free zinc air batteries alternatives Mercury free versions are commercially available for all applications of the main types of button cells (lithium, silver, oxide, alkaline and zinc air). The most frequently used types make use of zinc air technology (European Commission, 2014). Since October 2015, mercury-containing button cell batteries have been prohibited in the EU following the expiry of the exemption granted under the Batteries Directive. 1 Canada Alternatives: mercury-free silver oxide batteries, mercury-free zinc air batteries, lithium batteries Mercury-free alternatives have been available from major battery manufacturers since the late 1990s and early 2000s (e.g. -

Powering Biomedical Devices.Pdf
CHAPTER 11 Introduction The increase of world population is a challenge itself for world resources. The sustainability of food supplies, energy resources, and the environment are being questioned by analysts, while climate change just adds more pressure to the equation. The life expectancy of the world as a whole is rising while the fertility rate is declining. This will create a challenge in health care for the ageing population (Gavrilov and Heuveline, 2003). The United States alone will have 20% of the population over the age of 65 by 2050. In contrast, Europe will see rates close to 30% while Japan will arise to almost 40%, as summarized in Table 1.1. It is anticipated that in the near future, specialized health-care services will be in higher demand due to this increase. This demand will be characterized by medical resources not only to attend to this segment of the population, but also to keep them active as well. Therefore, the monitoring of physiological responses as well as specialized drug or other therapy delivery applications will be needed for portable, wearable, or implantable biomedical autonomous devices. In addition, wireless communication promises new medical applications such as the use of wireless body sensor networks for health monitoring (Jovanov et al., 2005; Hao and Foster, 2008; Varshney, 2007). These biomedical devices, however, come with their own issues, mainly power source challenges. Batteries are commonly used to energize most of these applications, but they have a finite lifetime. As biomedical Table 1.1 Percentage of Population Over 65 Years Olda Region 1950 2000 2050 World 5.2 6.8 16.2 USA 8.3 12.4 21.6 Europe 8.2 14.8 27.4 Japan 4.9 17.2 37.8 aPopulation Division of the Department of Economic and Social Affairs of the United Nations Secretariat, World Population Prospects: The 2008 Revision, http://esa.un.org/unpp. -

Button Cell Cr2032
BUTTON CELL CR2032 BRIEF SPECIFICATION Model: CR2032 Nominal Voltage: 3.0V Nominal Capacity:210mAh Standard Discharge with load: 15KΩ Weight: 3.0g Stainless steel container ISO9001 Certified UL Certified MH20555 Manufacturer: EEMB Co., Ltd. Website: http://eemb.com CR2032 Datasheet EEMB Lithium Manganese Dioxide Battery (Edition Sep.2014) Lithium Coin battery structure Note: Any representations in this brochure concerning performance, are for informational purposes only and are not construed as warranties either expressed or implied, of future performance. CR2032 Datasheet EEMB Lithium Manganese Dioxide Battery (Edition Sep.2014) EEMB CR2032 Button Cell STANDARD SPECIFICATION CONTENT 1. SUBJECT 2. GENERAL FEATURES AND APPLICATIONS 3. GENERAL SPECIFICATIONS 4. PERFORMANCE AND TEST METHODS 5. VISUAL ASPECT 6. PRECAUTIONS IN USING 7. STORAGE AND MOUNT 8. SAFETY 9. BATTERY CHARACTERISTICS 10. UNTAGGED CELL DIMENSIONS 11. MEMORY BACKUP CIRCUIT DESIGN SUGGESTION --------------------------------------------------------------------------------------------------------------------------------------------- 1. SUBJECT This specification presents typical and guaranteed ex-work values of the Lithium Manganese Dioxide Button Cells (Li / MnO2), of Model CR2032-PEN3 Manganese dioxide (MnO2) is used for the active cathode material, and high voltage, high activity lithium metal for the anode material. Battery discharge reactions are as follows: Anode reaction: Li Li+ + e- (IV) + - (III) Cathode reaction: Mn O2 + Li + e Mn O2 (Li+) (IV) + (III) Total reaction: Mn O2 + Li Mn O2 (Li+) 2. LI-MnO2 BUTTON CELL FEATURES AND APPLICATIONS Features: ¾ Light Weight, High Voltage and High Energy Density ¾ Excellent Stable Discharge Characteristics ¾ Outstanding Temperature Characteristics ¾ Excellent Leakage Resistance ¾ Excellent Long-term Reliability Note: Any representations in this brochure concerning performance, are for informational purposes only and are not construed as warranties either expressed or implied, of future performance. -

Battery Disposal
Online information about proper To find the nearest retail location to household battery management recycle your rechargeable batteries visit SCMUA’s www.rbrc.org. www.njhazwaste.com For More information on how to properly dispose of your Household Batteries, contact Guide to Proper www.rbrc.org your Municipal Recycling Coordinator: www.epa.gov/epawaste/conserve/ Household Town Phone Battery Andover Borough (973) 786-6688 Andover Township (973) 383-4280 x224 Management Battery Disposal Guidelines Branchville (973) 948-4626 Byram (973) 347-2500 x125 Battery Type Sizes Proper Frankford (973) 948-4230 Available Disposal Franklin (973) 827-9280 x100 Fredon (973) 383-7025 x0 Alkaline AAA, AA, Trash or SCMUA Green (908) 852-9333 x11 (Single Use) C, D, 6V, for disposal 9V,1.5V (Non-hazardous) Hamburg (973) 827-9230 x13 Hampton (973) 383-5570 Rechargeable AAA, AA, Terminals taped or Hardyston (973) 827-3525 C, D, 6V, bagged separately Hopatcong (973)398-3611 Ni-Cd 9V Ni-MH SCMUA or Lafayette (973) 383-1817 Ni-Zn www.rbrc.org Montague (973) 293-7300 Li-ion Newton (973) 383-3521 x226 Rechargeable Multiples of Terminals taped or 2 Volts; 2V, bagged separately Ogdensburg (973) 827-3712 Sealed Lead 6V, 12V Sandyston (973) 948-3520 x200 (Pb) Acid - SCMUA or Less than www.rbrc.org Sparta (973) 729-6174 PROCESSING BATTERIES 11 lbs Stanhope (973) 347-6368 Button Sizes vary Terminals taped or Alkaline (Single Use Batteries) bagged separately Stillwater (973) 383-8722 Rechargeable Batteries SCMUA Hazardous Sussex Borough (973) 875-4202 Waste Day Other Hazardous Batteries Vernon (973) 764-3021 Lithium 3V, 6V, 3V Terminals taped or button bagged separately Walpack (908) 841-9576 Update April 1, 2011 SCMUA Hazardous Wantage (973) 875-7192 Waste Day www.scmua.org or (973) 579-6998 Printed on Recycled Paper The Sussex County MUA has been WHAT TYPES OF BATTERIES collecting household batteries for SHOULD I RECYCLE? To find a retail collection site use the collection site locator at recycling and proper disposal since Recycle all www.call2recycle.org or call the the early 1990s. -

Appendix: Battery Standards
Appendix: Battery Standards The International Electrochemical Commission battery. These characteristics are: (lEC) have prepared a battery standards speci a) Dimensions and terminals (physical inter fication: lECPublication 86: Primary Cel/sand changeability) Batteries , parts 1 and 2, 1975. The relevant b) Voltage and electrical performance (elec British Standard is BS 397. Both are available trical interchangeability) from the British Standards Institution, 2 Park Street, London WlA 2BS. At the present time there are three possible The American National Standard, ANSI sources of such information. C18.l, is available from ANSI, 1430 Broadway, The battery manufacturer publishes litera New York 10018. ture describing his products and sometimes The Japanese Standards Association issues includes recommendations for the battery to Standard 115 8501 Dry Cellsand Batteries; 115 be used in specific equipment. In the nature 8508 Mercury Cells and Batteries ; 115 8509 of things this information is not complete. Alkaline-Manganese Dioxide Cells and Bat The equipment manufacturer may include teries ;115 8510 SilverOxide Cellsand Batteries; in the equipment a label stating the specific 115 8511 Alkaline Primary Cells and Batteries. type of battery to be used,or the size required. All are available through Japanese Embassies Often a more detailed list of approved bat or direct from the Japanese Standards Associ teries is given in the equipment instruction ation, 1-24 Akasaka 4 Chome , Minato-ku, manual but again this islikely to be incomplete. Tokyo. Furthermore the information may lead to The following is an extract from The lEC frustration when the equipment originates in System of Battery Designation, prepared by a different country from that in which the IEC Technical Committee No. -

PDF Downloads
International Journal of Clinical and Experimental Medicine Research, 2021, 5(3), 297-303 http: //www.hillpublisher.com/journals/ijcemr/ ISSN Online: 2575-7970 ISSN Print: 2575-7989 Button Batteries and Child Deaths: Market Failure of Unsafe Products John Paull University of Tasmania, Hobart, Tasmania, Australia. How to cite this paper: John Paull. (2021) Abstract Button Batteries and Child Deaths: Market Failure of Unsafe Products. International Button batteries can be lethal if ingested and not promptly excreted or re- Journal of Clinical and Experimental moved. These batteries are present in a multitude of household items including Medicine Research, 5(3), 297-303. car remotes, IT devices, and many consumer items targeted at children. There DOI: 10.26855/ijcemr.2021.07.011 are various technologies available for button batteries and they are produced Received: April 26, 2021 in various sizes. Lithium button batteries with a diameter of 20mm or more Accepted: May 22, 2021 pose the greatest risk. Most at risk are children of 0-5 years. At this age, there Published: June 15, 2021 is a propensity to put foreign objects in the mouth. These batteries once swal- *Corresponding author: John Paull, lowed, rather than passing through the alimentary canal and being excreted, University of Tasmania, Hobart, Tas- may lodge in the oesophagus where they will burn through tissue, sometimes mania, Australia. Email: [email protected] trough to the trachea, sometimes through to the aorta. This will be fatal for the child if the problem is not promptly identified and the battery then promptly extracted. Death will follow within several weeks of ingestion, following epi- sodes of ill health, if the problem is not recognised. -

Material Safety Datasheet (MSDS) Li-Ion Button Cell Battery, Model CR2032, Used in PRA-SCL (F.01U.325.042) PRA-SCM (F.01U.325.041) PRA-SCS (F.01U.325.040)
Material Safety Datasheet (MSDS) Li-ion button cell battery, model CR2032, used in PRA-SCL (F.01U.325.042) PRA-SCM (F.01U.325.041) PRA-SCS (F.01U.325.040) Multiple brands Gillette 37 A Street Environment Needham, MA 02492 Health and Safety Tel 781.292.8151 Page 1 of 4 MATERIAL SAFETY DATA SHEET NAME: DURACELL LITHIUM MANGANESE DIOXIDE COIN BATTERIES CAS NO: Not applicable Effective Date: 4/4/05 Rev: 4 A. — IDENTIFICATION % Formula: Mixture Mixture Manganese Dioxide (1313-13-9) 65-75 Molecular Weight: NA Propylene Carbonate (108-32-7) 10-15 Lithium (7439-93-2) 5-10 Synonyms: Lithium Manganese Dioxide Coin Cells: Graphite, synthetic (7440-44-0) 5-10 3V-DL2016; DL2025; DL2430; DL2450; 1,2-Dimethoxyethane (110-71-4) 1-10 DL2032; DL1616; DL1620 Lithium Perchlorate (7791-03-9) <1.5 B. — PHYSICAL DATA Boiling Point Melting Point Freezing Point NA °F NA °C NA °F NA °C NA °F NA °C Specific Gravity (H2O=1) Vapor Density (air=1) Vapor Pressure @ °F NA NA NA mm Hg Evaporation Saturation in Air Autoignition Temperature ( Ether =1) (by volume@ °F) °F °C NA NA NA % Volatiles Solubility in Water NA NA pH NA Appearance/Color Coin cells. Contents dark in color. Flash Point and Test Method(s) 1,2-Dimethoxyethane (Approximately 3-7% of contents): 42.8 °F, 6°C (Closed Cup) Flammable Limits in Air (% by volume) Lower NA % Upper NA % C. — REACTIVITY Stability X stable unstable Polymerization may occur X will not occur Conditions to Avoid Conditions to Avoid Do not heat, crush, disassemble, short circuit or Not applicable recharge. -

Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) Total of 30 Batteries
Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. Affordable Price Really good prices for Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. excellent Electronic and Accessory that you can purchase currently. See Product Image | Check Latest Price Now | Customer Reviews Lots of the customer opinions tell that the Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. is actually high quality Electronic and Accessory. It's a really very affordable product for the deal. Relax and take a several minute to read customer review articles, it cover product quality, features, bad and good for this product. All this information just might help you ordering safely, select a product spces that matches your wants and at a price you're ok. If you looking for great quality Electronic Accessory. Currently this site has the low price just for Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. now with good offers and super fast shipping available to you. Where to Order Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. Suitably? If you need to order Electronic Equipment at inexpensive, Amazon.com is perfect location which have a cheap price, it's perfect for every body who are are actually need to shopping on this Energizer Battery 357BPZ-3 Watch/Calculator Button Cell Battery with Zero Mercury - Card/3 (Pack of 10) total of 30 batteries. -
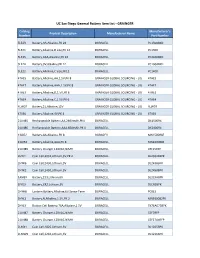
UC San Diego General Battery Item List - GRAINGER
UC San Diego General Battery Item List - GRAINGER Catalog Manufacturer's Product Description Manufacturer Name Number Part Number 5LE23 Battery,AA,Alkaline,PK 24 DURACELL PC1500BKD 5LE21 Battery,Alkaline,D size,PK 12 DURACELL PC1300 5LE25 Battery,AAA,Alkaline,PK 24 DURACELL PC2400BKD 5LE24 Battery,9V,Alkaline,PK 12 DURACELL PC1604BKD 5LE22 Battery,Alkaline,C Size,PK12 DURACELL PC1400 4TAE5 Battery,Alkaline,AA,1.5V,PK 8 GRAINGER GLOBAL SOURCING - LIG 4TAE5 4TAE7 Battery,Alkaline,AAA,1.5V,PK 8 GRAINGER GLOBAL SOURCING - LIG 4TAE7 4TAE3 Battery,Alkaline,D,1.5V,PK 6 GRAINGER GLOBAL SOURCING - LIG 4TAE3 4TAE4 Battery,Alkaline,C,1.5V,PK 6 GRAINGER GLOBAL SOURCING - LIG 4TAE4 4LW07 Battery,21,Alkaline,12V GRAINGER GLOBAL SOURCING - LIG 4LW07 4TAE6 Battery,Alkaline,9V,PK 4 GRAINGER GLOBAL SOURCING - LIG 4TAE6 21LN85 Rechargeable Battery,AA,2400mAh,PK4 DURACELL DX1500R4 21LN86 Rechargeable Battery,AAA,850mAh,PK 4 DURACELL DX2400R4 13J052 Battery,AA,Alkaline,PK 8 DURACELL MN1500B8Z 13J054 Battery,Alkaline,AAA,PK 8 DURACELL MN2400B8Z 21LN89 Battery Charger,120VAC,NiMH DURACELL CEF15RFP 2HYJ1 Coin Cell,2032,Lithium,3V,PK 2 DURACELL DL2032B2PK 2HYK6 Coin Cell,2430,Lithium,3V DURACELL DL2430BPK 2HYK2 Coin Cell,2450,Lithium,3V DURACELL DL2450BPK 1ANB9 Battery,223,Lithium,6V DURACELL DL223ABPK 1FYE9 Battery,CR2,Lithium,3V DURACELL DLCR2BPK 2HYH8 Lantern Battery,Alkaline,6V,Screw Term DURACELL PC915 2HYL1 Battery,N,Alkaline,1.5V,PK 2 DURACELL MN9100B2PK 2HYL3 Button Cell Battery,76A,Alkaline,1.5V DURACELL PX76A675BPK 21LN87 Battery Charger,120VAC,NiMH -

Maplin-Electronics-1982-09-11.Pdf
SEPTEMBER-NOVEMBER 1982 pr PRICE 60p *SATELLITES IN COMMUNICATIONS AND BROADCASTING First in a brand new series. *TELEPHONE EXCHANGE With push-button or rotary dialling, up to 32 two-wire extensions, solid-state switching. *FREQUENCY COUNTER 8-digit with high reliability solid-state switching for mains or .12V operation. *ULTRASONIC INTRUDER DETECTOR Cover large areas with one simple, low-cost unit. *NICKEL CADMIUM CELLS Everything you always wanted to know. PLUS — Remote controller for amplifier, ZX81 PIO board, complete Maplin Price List DID YOU MISS THESE ISSUES? Copies of issue 1 are still available for just 60p, and include all these interesting projects: Universal Timer. A comprehensive programmable controller for up to 4 mains appliances. There is storage for up to 18 program times, ons or offs and relay outputs. Complete construction details. Combo Amplifier. Superb 120W MOSFET power amp with low-noise BI-FET pre-amp having built-in flanger, inputs for guitars, keyboards or microphones, and five step equaliser. Complete construction details. Temperature Gauge. Coloured LED indication of 10°C to 100°C. Complete construction details. Pass The Bomb! Low-cost easy to build electronic version of pass-the-parcel — keeps the kids amused for hours! Plus six easy to build projects on Veroboard: Car Battery Monitor — Colour Snap Game — CMOS Logic Probe — Peak Level Indicator — Games Timer — Multi-Colour Pendant. Issue 1 also included features on Videotext and How To Solder and feature series, Basically BASIC, Starting Point and Circuit Maker. All this for just 60p. Order As XAO1B (Maplin Magazine Volume 1 No 1) Price 60pNV Copies of issue 2 are now sold out, but a reprint of the projects from issue 2 is available and contains: Digital Multi-Train Controller.