Contributions to the Knowledge of Neotropical Lycaenid Butterflies: Polyommatinae (Lepidoptera: Lycaenidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Read the Comments
CropLife * AMERICA * ~ March 2,2015 VIA FEDERAL E-RULEMAKING PORTAL Public Comments Processing Attn: FWS-R3-ES-2014-0056 U.S. Fish and Wildlife Service Headquarters MS: BPHC 5275 Leesburg Pike Falls Church, VA 22041-3803 Re: Initial Comments: 90-Day Finding on a Petition to List the Monarch Butterfly (Danaus Plexippus Plexippus) as Threatened Under the Endangered Species Act, 79 Fed. Reg. 78775 (Dec. 31, 2014) Dear Sir or Madam, CropLife America ("CropLife") is the national voice of the agricultural crop protection industry. CropLife represents companies that develop, manufacture, and distribute virtually all ofthe crop protection, pest management, and biotechnology products used by American farmers. Because these products are critical technologies for American agriculture, CropLife's members have a substantial interest in the issues presented in the 90-day finding on a petition to list as threatened the Monarch butterfly (Dana us plexippus p/exippus) made available to the publicI by the U.S. Fish and Wildlife Service ("FWS") on December 31, 2014 pursuant to the Endangered Species Act ("ESA,,). 2 As these initial comments indicate, our organization and its members believe that the proposed listing of the Monarch butterfly under the ESA is not warranted. Estimates for Monarch populations in North America have been available for only about two decades. CropLife and its members understand that overall estimated population levels in North America have declined during that period, although the data indicate that Monarch population numbers fluctuate very widely from year-to-year.3 Indeed, just this past year, the Eastern North I See Endangered and Threatened Wildlife and Plants; 90-Day Findings on Two Petitions, 79 Fed. -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -

Pine Island Ridge Management Plan
Pine Island Ridge Conservation Management Plan Broward County Parks and Recreation May 2020 Update of 1999 Management Plan Table of Contents A. General Information ..............................................................................................................3 B. Natural and Cultural Resources ...........................................................................................8 C. Use of the Property ..............................................................................................................13 D. Management Activities ........................................................................................................18 E. Works Cited ..........................................................................................................................29 List of Tables Table 1. Management Goals…………………………………………………………………21 Table 2. Estimated Costs……………………………………………………………….........27 List of Attachments Appendix A. Pine Island Ridge Lease 4005……………………………………………... A-1 Appendix B. Property Deeds………….............................................................................. B-1 Appendix C. Pine Island Ridge Improvements………………………………………….. C-1 Appendix D. Conservation Lands within 10 miles of Pine Island Ridge Park………….. D-1 Appendix E. 1948 Aerial Photograph……………………………………………………. E-1 Appendix F. Development Agreement………………………………………………….. F-1 Appendix G. Plant Species Observed at Pine Island Ridge……………………………… G-1 Appendix H. Wildlife Species Observed at Pine Island Ridge ……... …………………. H-1 Appendix -

Butterflies from the Middle Eocene: the Earliest Occurrence of Fossil Papilionoidea (Lepidoptera)
THE PEARCE- SELLARDS Sctks NUMBER 29 BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) Christopher J. Durden and Hugh Rose 1978 Texas Memorial Museum/2400 Trinity/Austin, Texas 78705 W. W. Newcomb, Director The Pearce-Sellards Series is an occasional, miscellaneous series of brief reports of museum and museum associated field investigations and other research. Its title seeks to commemorate the first two directors of the Texas Memorial Museum, now both deceased: J. E. Pearce and Dr. E. H. Sellards, professors of anthropology and geology respectively, of The University of Texas. A complete list of Pearce-Sellards papers, as well as other publica- tions of the museum, will be sent upon request. BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) 1 Christopher J. Durden 2 and Hugh Rose 3 ABSTRACT Three fossil butterflies recently collected from the Green River Shale of Colorado extend the known range of Rhopalocera eight to ten million years back, to 48 Ma. Praepapilio Colorado n. g., n. sp., and P. gracilis n. sp. are primitive Papilionidae related to the modern Baronia brevicornis Salvin, but they require a new subfamily, Praepapilioninae. Riodinella nympha n. g., n. sp. is a primitive member of the Lycaenidae, related to modern Ancyluris, Riodina, and Rhetus, in the tribe Riodinidi. INTRODUCTION With approximately 194,000 living species, the Lepidoptera is, after the Coleoptera with some 350,000, species, the second most diverse order of organisms. It is underrepresented in the fossil record (Scudder 1875, 1891, 1892; Handlirsch 1925;Mackay 1970;Kuhne 1973; Shields 1976). -

Pacific Islands Area
Habitat Planting for Pollinators Pacific Islands Area November 2014 The Xerces Society for Invertebrate Conservation www.xerces.org Acknowledgements This document is the result of collaboration with state and federal agencies and educational institutions. The authors would like to express their sincere gratitude for the technical assistance and time spent suggesting, advising, reviewing, and editing. In particular, we would like to thank the staff at the Hoolehua Plant Materials Center on the Hawaiian Island of Molokai, NRCS staff in Hawaii and American Samoa, and researchers and extension personnel at American Samoa Community College Land Grant (especially Mark Schmaedick). Authors Written by Jolie Goldenetz-Dollar (American Samoa Community College), Brianna Borders, Eric Lee- Mäder, and Mace Vaughan (The Xerces Society for Invertebrate Conservation), and Gregory Koob, Kawika Duvauchelle, and Glenn Sakamoto (USDA Natural Resources Conservation Service). Editing and layout Ashley Minnerath (The Xerces Society). Updated November 2014 by Sara Morris, Emily Krafft, and Anne Stine (The Xerces Society). Photographs We thank the photographers who generously allowed use of their images. Copyright of all photographs remains with the photographers. Cover main: Jolie Goldenetz-Dollar, American Samoa Community College. Cover bottom left: John Kaia, Lahaina Photography. Cover bottom right: Gregory Koob, Hawaii Natural Resources Conservation Service. Funding This technical note was funded by the U.S. Department of Agriculture (USDA) Natural Resources Conservation Service (NRCS) and produced jointly by the NRCS and The Xerces Society for Invertebrate Conservation. Additional support was provided by the National Institute for Food and Agriculture (USDA). Please contact Tony Ingersoll ([email protected]) for more information about this publication. -

FM), 3-9 July, 3-10 September and 10-13 December 1990
BULLETIN OF THE ALLYN MUSEUM 3621 Bayshore Rd. Sarasota, Florida 34234 Published By Florida Museum of Natural History University of Florida Gainesville, Florida 32611 Number 133 14 June 1991 ISSN-0097-3211 THE BUTTERFLIES OF ANEGADA, BRITISH VIRGIN ISLANDS, WITH DESCRIPTIONS OF A NEW CALISTO (SATYRIDAE) AND A NEW COPAEODES (HESPERIIDAE) ENDEMIC TO THE ISLAND David Spencer Smith Hope Entomological Collections, The University Museum, Parks Road, Oxford, OX! 3PW, England. Lee D. Miller Allyn Museum of Entomology of the Florida Museum of Natural History, 3621 Bay Shore Road, Sarasota, Florida 34234, U.S.A. Faustino KcKenzie Institute of Neurobiology, University of Puerto Rico, Boulevard del Valle 201, Old San Juan, Puerto Rico 00901, U.S.A. This paper is dedicated to the memory of John Griffith of Jesus College, Oxford. INTRODUCTION Anegada island is the northernmost member of the Lesser Antillean arc, situated at 18" 43'N and 64" 19'W. Its nearest neighbors are Anguilla, about 80 statute miles (127 km} across the Anegada Passage to the east-southeast and Virgin Gorda, about 13 miles (21 km} due south. Whereas the Virgin Islands are generally mountainous, Anegada reaches perhaps 18 ' above mean sea level and much of the island is considerably lower (D 'Arcy, 1975}. It is about 10 miles (16 km} in length, about 15 square miles (39 km'} in area, oriented along the east-west axis and is just over 2 miles (3.5 km} across the widest point (Fig. 16}. From the south coast and into the Anegada Passage to the southeast extends the Horseshoe Reef, long a hazard to navigation. -

Redalyc.On the Recent Invasion of the Canary Islands by Two Butterfly
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Wiemers, M.; Acosta-Fernández, B.; Larsen, T. B. On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) SHILAP Revista de Lepidopterología, vol. 41, núm. 161, marzo, 2013, pp. 95-104 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45528755006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 95-104 On the recent invasion o 10/3/13 18:45 Página 95 SHILAP Revta. lepid., 41 (161), marzo 2013: 95-104 CODEN: SRLPEF ISSN: 0300-5267 On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) M. Wiemers, B. Acosta-Fernández & T. B. Larsen Summary Leptotes pirithous is reported from Gran Canaria for the first time, the recent spreading of this species and of Cacyreus marshalli in the Macaronesian Islands is discussed, and distribution maps of both species are presented for the Canary Islands. KEY WORDS: Lepidoptera, Lycaenidae, Leptotes pirithous, Gran Canaria, Spain. Sobre la reciente invasion de dos mariposas en las Islas Canarias con el primer registro de Leptotes pirithous (Linnaeus, 1767) de Gran Canaria, España (Lepidoptera: Lycaenidae) Resumen Se registra por primera vez para Gran Canaria a Leptotes pirithous, se discute la reciente expansión de esta especie y de Cacyreus marshalli en Macaronesia y se dan mapas de distribución de ambas especies presentes en las Islas Canarias. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Who Killed All the Miami Blues? by Dennis Olle Holly Salvato
James L. Monroe Who Killed All the Miami Blues? by Dennis Olle Holly Salvato The night of the iguana. Populations of non-native introduced iguanas have exploded on the Florida Keys. Nickerbeans, the caterpillar foodplant for Miami Blues on Bahia Honda, are part of their diet. Aug. 26, 2010. Bahia Honda State Park, FL. The re-discovery and would-be protection and restoration of Miami Blues in Florida has been Miami Blues have disappeared — the well-chronicled in these pages (see below). I U.S. Fish & Wildlife Service in the wish I had better news to report regarding the G. W. Bush administrations failed status of this rare butterfly, but I do not. to declare them an endangered While sitting in a parking lot in El Cerrito, species; the State of Florida, despite California checking my office emails, I received good intentions, failed to implement word from a representative of the Florida Fish a management plan; and personnel and Wildlife Commission to the effect that: The at University of Florida failed to learn “flagship” wild Miami Blue colony at Bahia what factors have caused their decline Honda State Park in the Lower Florida Keys had or to maintain the laboratory colony apparently collapsed (in fact, neither adults nor These mated Miami Blues, to the best of our knowledge the last Miami Blues created as a safety valve if disaster caterpillars have been seen at Bahia Honda seen at Bahia Honda State Park, provided hope for a future that has now died. befell the Bahia Honda colony. State Park since January 2010) and the captive Jan. -
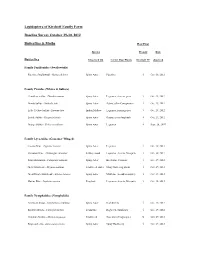
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Joel Carreiro. Seeing Things
JOEL CARREIRO. SEEING THINGS by Elinor Richter Ultimately seeing alters the thing that is seen and transforms the seer. Seeing is metamorphosis, not mechanism.' - James Elkins, The Object Stares Back When we concentrate on a material object, whatever its situation, the very act of attention may lead to our involuntarily sinking into the history of that object. Novices must learn to skim over matter if they want to stay at the exact level of the moment. Transparent things, through which the past shines!2 - Vladimir Nabokov, Transparent Things Joel Carreiro's favorite writer is Vladimir Nabokov (1899-1977). Several of his works refer directly to the Russian-American author's output: Pninian (2007), Zemblan (2008), Harlequin (2009), Atalanta (2010) and Icebergs in Paradise (2008).3 In a recent article in the New York Review of Books, John Banville partially explains the appeal of Nabokov, this most elegant of stylists: He does tell a wonderful story, he does teach us many subtle and intricate things, he does ultimately enchant. Yet when we press past the surface dazzle of his work—no small feat—we find ourselves in a world as strange and yet strangely familiar as the one into which Alice stepped through the looking glass.' Carreiro is similarly an enchanter by " the bringing back in changed form of things already known; as the defamiliarization of the familiar."' Nabokov, Carreiro, and ultimately Banville himself transport their viewers into a realm that is at once familiar and yet somehow different. Nabokov is best known as an author for his complex plots and clever wordplays as well as for the incredible diversity of his interests which ranged from Pushkin, to chess, to tennis, to entomology, especially the study of Lepidoptera.6 Carreiro's search for imagery has led him to such varied sources as old master paintings, Meissen figurines based on commedia dell'arte characters, medieval manuscripts, Mughal miniatures, and early zoological illustrations.' Carreiro "cannibalizes" the entire spectrum of art history. -

BUTTERFLIES in Thewest Indies of the Caribbean
PO Box 9021, Wilmington, DE 19809, USA E-mail: [email protected]@focusonnature.com Phone: Toll-free in USA 1-888-721-3555 oror 302/529-1876302/529-1876 BUTTERFLIES and MOTHS in the West Indies of the Caribbean in Antigua and Barbuda the Bahamas Barbados the Cayman Islands Cuba Dominica the Dominican Republic Guadeloupe Jamaica Montserrat Puerto Rico Saint Lucia Saint Vincent the Virgin Islands and the ABC islands of Aruba, Bonaire, and Curacao Butterflies in the Caribbean exclusively in Trinidad & Tobago are not in this list. Focus On Nature Tours in the Caribbean have been in: January, February, March, April, May, July, and December. Upper right photo: a HISPANIOLAN KING, Anetia jaegeri, photographed during the FONT tour in the Dominican Republic in February 2012. The genus is nearly entirely in West Indian islands, the species is nearly restricted to Hispaniola. This list of Butterflies of the West Indies compiled by Armas Hill Among the butterfly groupings in this list, links to: Swallowtails: family PAPILIONIDAE with the genera: Battus, Papilio, Parides Whites, Yellows, Sulphurs: family PIERIDAE Mimic-whites: subfamily DISMORPHIINAE with the genus: Dismorphia Subfamily PIERINAE withwith thethe genera:genera: Ascia,Ascia, Ganyra,Ganyra, Glutophrissa,Glutophrissa, MeleteMelete Subfamily COLIADINAE with the genera: Abaeis, Anteos, Aphrissa, Eurema, Kricogonia, Nathalis, Phoebis, Pyrisitia, Zerene Gossamer Wings: family LYCAENIDAE Hairstreaks: subfamily THECLINAE with the genera: Allosmaitia, Calycopis, Chlorostrymon, Cyanophrys,