Notice of Investigation Shall Be Served
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Produzent Adresse Land Allplast Bangladesh Ltd
Zeitraum - Produzenten mit einem Liefertermin zwischen 01.01.2020 und 31.12.2020 Produzent Adresse Land Allplast Bangladesh Ltd. Mulgaon, Kaliganj, Gazipur, Rfl Industrial Park Rip, Mulgaon, Sandanpara, Kaligonj, Gazipur, Dhaka Bangladesh Bengal Plastics Ltd. (Unit - 3) Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh HKD International (Cepz) Ltd. Plot # 49-52, Sector # 8, Cepz, Chittagong Bangladesh Lhotse (Bd) Ltd. Plot No. 60 & 61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Plastoflex Doo Branilaca Grada Bb, Gračanica, Federacija Bosne I H Bosnia-Herz. ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Konrrg Pisey, Kampong Spueu Cambodia Powerjet Home Product (Cambodia) Co., Ltd. Manhattan (Svay Rieng) Special Economic Zone, National Road 1, Sangkat Bavet, Krong Bavet, Svaay Rieng Cambodia AJS Electronics Ltd. 1st Floor, No. 3 Road 4, Dawei, Xinqiao, Xinqiao Community, Xinqiao Street, Baoan District, Shenzhen, Guangdong China AP Group (China) Co., Ltd. Ap Industry Garden, Quetang East District, Jinjiang, Fujian China Ability Technology (Dong Guan) Co., Ltd. Songbai Road East, Huanan Industrial Area, Liaobu Town, Donggguan, Guangdong China Anhui Goldmen Industry & Trading Co., Ltd. A-14, Zongyang Industrial Park, Tongling, Anhui China Aold Electronic Ltd. Near The Dahou Viaduct, Tianxin Industrial District, Dahou Village, Xiegang Town, Dongguan, Guangdong China Aurolite Electrical (Panyu Guangzhou) Ltd. Jinsheng Road No. 1, Jinhu Industrial Zone, Hualong, Panyu District, Guangzhou, Guangdong China Avita (Wujiang) Co., Ltd. No. 858, Jiaotong Road, Wujiang Economic Development Zone, Suzhou, Jiangsu China Bada Mechanical & Electrical Co., Ltd. No. 8 Yumeng Road, Ruian Economic Development Zone, Ruian, Zhejiang China Betec Group Ltd. -

China Year Book 2011
CHINA YEAR BOOK 2011 Edited by BRIGADIER MANDIP SINGH, VSM 1 CHINA YEAR BOOK Cover map not to scale. Institute for Defence Studies and Analyses, New Delhi. All rights reserved. No part of this publication may be reproduced, sorted in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photo-copying, recording or otherwise, without the prior permission of the Institute for Defence Studies and Analyses (IDSA). ISBN: 978-93-82169-04-8 Disclaimer: The views expressed in this Report are of the Task Force Members and do not necessarily reflect those of the Institute for Defence Studies and Analyses or the Government of India. First Published: May 2012 Price: Rs. 299/- Published by: Institute for Defence Studies and Analyses No.1, Development Enclave, Rao Tula Ram Marg, Delhi Cantt., New Delhi - 110 010 Tel. (91-11) 2671-7983 Fax.(91-11) 2615 4191 E-mail: [email protected] Website: http://www.idsa.in Layout & Cover by: Vaijayanti Patankar Printed at: M/s Printline H-10, IInd Floor, NDSE-I New Delhi - 110049 Tel: (91-11) 24651060, 24643119 Mob: 9716335119 Email: [email protected] 2 CONTENTS Foreword ............................................................................. 5 Introduction ......................................................................... 7 Section I: External Relations 1. Sino-Indian Relations in 2011: Two Steps Forward, One Step Backward ................ 13 Rup Narayan Das 2. China's Current Central Asia Policy: Revisiting Priorities ................................................... 24 Jagannath P Panda 3. China and South Asia: Dragon Displacing the Elephant? ............................ 35 South Asia Centre 4. China-US Relations in 2011: Stymied by Strategic Mistrust ................................. 55 Rukmani Gupta 5. China and ASEAN in 2011: Redefining a Relationship ........................................ -

Transport, Vertical Structure and Radiative Properties of Dust Events in Southeast China Determined from Ground and Space Sensors
Atmospheric Environment 45 (2011) 6469e6480 Contents lists available at ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Transport, vertical structure and radiative properties of dust events in southeast China determined from ground and space sensors Jianjun Liu a,b, Youfei Zheng a, Zhanqing Li a,b,*, Connor Flynn c, E.J. Welton d, Mareen Cribb b a Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, Nanjing University of Information Science & Technology, Nanjing, China b Department of Atmospheric and Oceanic Science and Earth System Science Interdisciplinary Center, University of Maryland, College Park, Maryland, USA c Pacific Northwest National Laboratory, Richland, Washington, USA d Goddard Space Flight Center, NASA, Greenbelt, Maryland, USA article info abstract Article history: Two dust events were detected over the Yangtze Delta region of China during March 14e17 and April 25e26 Received 4 March 2011 in 2009 where such dust events are uncommon. The transport behavior, spatio-temporal evolution, vertical Accepted 11 April 2011 structure, direct radiative effects, as well as induced heating rates, are investigated using a combination of ground-based and satellite-based measurements, a back-trajectory analysis, an aerosol model and a radiative Keywords: transfer model. Back-trajectories, wind fields and aerosol model analyses show that the first dust originated Dust in northern/northwestern China and the second generated in the Taklimakan desert in northwest China, and Transport traveled across the Hexi corridor and Loess Plateau to the Yangtze Delta region (the so-called “dust corridor”). Vertical structure Æ Radiative properties The mean lidar extinction-to-backscatter ratio (LR) during the two dust events was 38.7 10.4 sr and Æ d fi Æ Southeast China 42.7 15.2 sr, respectively. -

List of Approved COVID-19 Rapid Antibody Test Kits As of 16 April 2020, 4:00PM
Republic of the Philippines Department of Health FOOD AND DRUG ADMINISTRATION List of Approved COVID-19 Rapid Antibody Test Kits as of 16 April 2020, 4:00PM NO. PRODUCT NAME MANUFACTURER 1 NANJING VAZYME 2019-nCoV Biolidics Limited. – 37 Jalan Pemimpin, #02-07, IgG/IgM DETECTION KIT Mapex, Singapore 2 NOVEL CORONAVIRUS (2019- NCOV) IgM/IgG ANTIBODY Nanjing Vazyme Medical Technology Co., Ltd – Level DETECTION KIT (COLLOIDAL 1-3, Bldg. C2, Red Maple Sci-Tech Park, Kechuang GOLD METHOD) Road, Nanjing China 3 DIAGNOSTIC KIT FOR IgM/IgG Zhuhai Livzon Diagnostic Inc. – 1st Building, No. 266, ANTIBODY TO CORONAVIRUS Tongchang Road, Xiangzhou District, Zhuhai, (SARS-CoV-2) (COLLOIDAL GOLD) Guangdong Province, People’s Republic of China 4 Innovita (Tangshan) Biological Technology Co., Ltd. – 2019-nCoV ANTIBODY TEST No. 699 Juxin Street, High-Tech Industrial (COLLOIDAL GOLD) Development Zone, Qian’an 064400, Hebei, China 5 SARS-CoV-2 ANTIBODY TEST Guangzhou Wondfo Biotech Co., Ltd. – No. 8 (LATERAL FLOW METHOD) Lizhishan Road, Science City, Luogang District, 510663, Guangzhou, People’s Republic of China 6 WANTAI SARS- CoV Ab RAPID TEST Beijing Wantai Biological Pharmacy Enterprise Co., KIT Ltd. – 31 Kexueyuan Road, Changping District Beijing, People’s Republic of China 7 Hangzhou Alltest Biotech Co., Ltd. – #550, Yinhai 2019 n-CoV IgG/IgM RAPID TEST Street, Hangzhou Economy and Technology CASSETTE Development Area, 310018, Hangzhou, People’s Republic of China Civic Drive, Filinvest City, Alabang 1781 Muntinlupa, Philippines Trunk Line +63 2 857 1900 Fax +63 2 807 0751 Website: www.fda.gov.ph Email: [email protected] Republic of the Philippines Department of Health FOOD AND DRUG ADMINISTRATION NO. -

Shop Direct Factory List Dec 18
Factory Factory Address Country Sector FTE No. workers % Male % Female ESSENTIAL CLOTHING LTD Akulichala, Sakashhor, Maddha Para, Kaliakor, Gazipur, Bangladesh BANGLADESH Garments 669 55% 45% NANTONG AIKE GARMENTS COMPANY LTD Group 14, Huanchi Village, Jiangan Town, Rugao City, Jaingsu Province, China CHINA Garments 159 22% 78% DEEKAY KNITWEARS LTD SF No. 229, Karaipudhur, Arulpuram, Palladam Road, Tirupur, 641605, Tamil Nadu, India INDIA Garments 129 57% 43% HD4U No. 8, Yijiang Road, Lianhang Economic Development Zone, Haining CHINA Home Textiles 98 45% 55% AIRSPRUNG BEDS LTD Canal Road, Canal Road Industrial Estate, Trowbridge, Wiltshire, BA14 8RQ, United Kingdom UK Furniture 398 83% 17% ASIAN LEATHERS LIMITED Asian House, E. M. Bypass, Kasba, Kolkata, 700017, India INDIA Accessories 978 77% 23% AMAN KNITTINGS LIMITED Nazimnagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 1708 60% 30% V K FASHION LTD formerly STYLEWISE LTD Unit 5, 99 Bridge Road, Leicester, LE5 3LD, United Kingdom UK Garments 51 43% 57% AMAN GRAPHIC & DESIGN LTD. Najim Nagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 3260 40% 60% WENZHOU SUNRISE INDUSTRIAL CO., LTD. Floor 2, 1 Building Qiangqiang Group, Shanghui Industrial Zone, Louqiao Street, Ouhai, Wenzhou, Zhejiang Province, China CHINA Accessories 716 58% 42% AMAZING EXPORTS CORPORATION - UNIT I Sf No. 105, Valayankadu, P. Vadugapal Ayam Post, Dharapuram Road, Palladam, 541664, India INDIA Garments 490 53% 47% ANDRA JEWELS LTD 7 Clive Avenue, Hastings, East Sussex, TN35 5LD, United Kingdom UK Accessories 68 CAVENDISH UPHOLSTERY LIMITED Mayfield Mill, Briercliffe Road, Chorley Lancashire PR6 0DA, United Kingdom UK Furniture 33 66% 34% FUZHOU BEST ART & CRAFTS CO., LTD No. 3 Building, Lifu Plastic, Nanshanyang Industrial Zone, Baisha Town, Minhou, Fuzhou, China CHINA Homewares 44 41% 59% HUAHONG HOLDING GROUP No. -
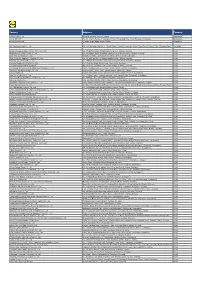
Factory Address Country
Factory Address Country Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh Lhotse (BD) Ltd. Plot No. 60&61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Bengal Plastics Ltd. Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Korng Pisey District, Konrrg Pisey, Kampong Speu Cambodia Ningbo Zhongyuan Alljoy Fishing Tackle Co., Ltd. No. 416 Binhai Road, Hangzhou Bay New Zone, Ningbo, Zhejiang China Ningbo Energy Power Tools Co., Ltd. No. 50 Dongbei Road, Dongqiao Industrial Zone, Haishu District, Ningbo, Zhejiang China Junhe Pumps Holding Co., Ltd. Wanzhong Villiage, Jishigang Town, Haishu District, Ningbo, Zhejiang China Skybest Electric Appliance (Suzhou) Co., Ltd. No. 18 Hua Hong Street, Suzhou Industrial Park, Suzhou, Jiangsu China Zhejiang Safun Industrial Co., Ltd. No. 7 Mingyuannan Road, Economic Development Zone, Yongkang, Zhejiang China Zhejiang Dingxin Arts&Crafts Co., Ltd. No. 21 Linxian Road, Baishuiyang Town, Linhai, Zhejiang China Zhejiang Natural Outdoor Goods Inc. Xiacao Village, Pingqiao Town, Tiantai County, Taizhou, Zhejiang China Guangdong Xinbao Electrical Appliances Holdings Co., Ltd. South Zhenghe Road, Leliu Town, Shunde District, Foshan, Guangdong China Yangzhou Juli Sports Articles Co., Ltd. Fudong Village, Xiaoji Town, Jiangdu District, Yangzhou, Jiangsu China Eyarn Lighting Ltd. Yaying Gang, Shixi Village, Shishan Town, Nanhai District, Foshan, Guangdong China Lipan Gift & Lighting Co., Ltd. No. 2 Guliao Road 3, Science Industrial Zone, Tangxia Town, Dongguan, Guangdong China Zhan Jiang Kang Nian Rubber Product Co., Ltd. No. 85 Middle Shen Chuan Road, Zhanjiang, Guangdong China Ansen Electronics Co. Ning Tau Administrative District, Qiao Tau Zhen, Dongguan, Guangdong China Changshu Tongrun Auto Accessory Co., Ltd. -

11 Environmental Impact Assessment Framework of Scattered Rural Sewage Treatment Project
Public Disclosure Authorized Environmental Management Plan For Zhejiang Rural Water Supply and Sanitaion Project Public Disclosure Authorized Public Disclosure Authorized The Foreign Loan Supporting Project Leading Group Office of Zhejiang Province Public Disclosure Authorized MAY 2014 Environmental Management Plan for Zhejiang Rural Water Supply and Sanitaion Project Contents Preface ............................................................................................................................................. 1 1 Project Description ..................................................................................................................... 2 1.1 Project Background ......................................................................................................... 2 1.2 Project Constitution ......................................................................................................... 3 1.2.1 Source of fund ........................................................................................................ 4 2. Environmental Standards and Environmental Protection Objectives ................................ 19 2.1 Basis of Compilation ...................................................................................................... 19 2.1.1 Laws, Regulations and Rules ............................................................................. 19 2.1.2 Relevant regulations made by World Bank ...................................................... 20 2.1.3 Main technical specifications, technical -

Federal Register/Vol. 86, No. 14/Monday, January 25, 2021/Notices
6918 Federal Register / Vol. 86, No. 14 / Monday, January 25, 2021 / Notices exclusion order, a cease and desist order Persons filing written submissions Issued: January 19, 2021. and impose a bond upon respondents’ must file the original document William Bishop, alleged infringing articles during the 60- electronically on or before the deadlines Supervisory Hearings and Information day Presidential review period pursuant stated above. Submissions should refer Officer. to 19 U.S.C. 1337(j). to the docket number (‘‘Docket No. [FR Doc. 2021–01504 Filed 1–22–21; 8:45 am] Proposed respondents, other 3525’’) in a prominent place on the BILLING CODE 7020–02–P interested parties, and members of the cover page and/or the first page. (See public are invited to file comments on Handbook for Electronic Filing any public interest issues raised by the Procedures, Electronic Filing INTERNATIONAL TRADE complaint or § 210.8(b) filing. Procedures 1). Please note the COMMISSION Comments should address whether Secretary’s Office will accept only issuance of the relief specifically electronic filings during this time. [Inv. No. 337–TA–1239] requested by the complainant in this Filings must be made through the Certain Gabapentin Immunoassay Kits investigation would affect the public Commission’s Electronic Document and Test Strips, Components Thereof, health and welfare in the United States, Information System (EDIS, https:// and Methods Therefor; Institution of competitive conditions in the United edis.usitc.gov.) No in-person paper- Investigation States economy, the production of like based filings or paper copies of any or directly competitive articles in the electronic filings will be accepted until AGENCY: U.S. -

Table of Contents
2020 IEEE 20th International Conference on Software Quality, Reliability and Security Companion (QRS-C) QRS-C 2020 Table of Contents Message from the Steering Committee Chairs and General Chairs xviii Message from the Program Chairs xix Organizing Committee xx Program Committee xxii Steering Committee xxv List of Additional Reviewers xxvi Chairs of Workshops Co-Located with QRS 2020 xxvii ICCLC-Committee xxix Automated and Intelligent Software Testing Metamorphic Relations Identification on Chebyshev Rational Approximation Method in the Nuclide Depletion Calculation Program 1 Meng Li (University of South China, China; CNNC Key Laboratory on High Trusted Computing, China), Lijun Wang (University of South China, China), Shiyu Yan (University of South China, China; CNNC Key Laboratory on High Trusted Computing, China), Xiaohua Yang (University of South China, China; CNNC Key Laboratory on High Trusted Computing, China), Jie Liu (University of South China, China), and Yapin Wan (University of South China, China) Online Prediction of Server Crash Based on Running Data 7 Zou Zhuoliang (Beihang University, China) and Ai Jun (Beihang University, China) Efficiency Metrics and Test Case Design for Test Automation 15 Davrondzhon Gafurov (Norsk Helsenett SF) and Arne Erik Hurum (Norsk Helsenett SF) Agent-Based Software Testing: A Definition and Systematic Mapping Study 24 Pavithra Perumal Kumaresen (Mälardalen University), Mirgita Frasheri (Mälardalen University), and Eduard Enoiu (Mälardalen University) Intelligent Radar Software Defect Prediction -

The Warehouse Ethical Sourcing Report 2021
ETHICAL SOURCING REPORT 2021 2 CONTENTS INTRODUCTION APPENDICES 04. CEO introduction 22. 2019 – 2020 KPI table 05. Programme at a glance 23. Factory policy poster 06. Top 20 source countries 24. Apparel tier 1 factory list 2020 SUMMARY 26. Apparel tier 2 factory list 08. 2020 summary 36. Apparel brand list 10. Her Project updates 37. Other categories factory list Getting the most from this report 12. COVID-19 updates 49. Country wage & working hour table A brief overview of our programme and progress in 2020 can be viewed on pages 5 and 8. Our key areas of focus 13. Responding to forced labour risks and achievements can be found on pages 10 to 20. SUSTAINABLE MATERIALS Finally, the appendices from page 22 reveal performance trends over the past two years, and feature our factory 16. Better Cotton Initiative policy poster, brand and factory lists and source country wage and working hour data. 17. Forest Stewardship Council OUR POLICY IN PRACTICE 19. Policy in practice ETHICAL SOURCING REPORT 2021 INTRODUCTION. ETHICAL SOURCING REPORT 2021 4 CEO’S INTRODUCTION. APPENDICES programme. I believe, given our scale and challenges and achievements to date, diversity, this is the leading programme as well the road ahead. We invite both of its kind within the New Zealand retail your encouragement and suggestions for Ethical Sourcing is just one programme sector. improvement. within The Warehouse’s suite of “Sustainable & affordable” initiatives. Within The Warehouse Group’s 2020 Finally, I want to express my sincere Sustainable & affordable is The POLICY IN PRACTICE POLICY Annual Report, I pledged that COVID-19 gratitude to our ethical sourcing Warehouse’s guiding statement and will not slow down our commitment specialists and their colleagues within branding device representing our to becoming one of New Zealand’s our 200 person strong sourcing team. -
And Coarse-Mode Aerosol Direct Radiative Forcing Over Regions in East and Southeast Asia Base
ORIGINAL RESEARCH https://doi.org/10.4209/aaqr.200503 Analysis of Aerosol Type and Fine- and Aerosol and Air Quality Coarse-mode Aerosol Direct Radiative Forcing Research over Regions in East and Southeast Asia Based on AERONET Version 3 Data 1 1,2* 1 3 4 Jianyu Lin , Xinyong Shen , Lizhu Xing , Huizheng Che , B.N. Holben 1 Key Laboratory of Meteorological Disaster, Ministry of Education/Joint International Research Laboratory of Climate and Environment Change/Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters, Nanjing University of Information Science and Technology, Nanjing 210044, China 2 Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai 519082, China 3 State Key Laboratory of Severe Weather (LASW) and Key Laboratory of Atmospheric Chemistry (LAC), Chinese Academy of Meteorological Sciences, CMA, Beijing 100081, China 4 Biospheric Sciences Branch, NASA Goddard Space Flight Center, Greenbelt, Maryland, USA ABSTRACT Based on the particle linear depolarization ratio (PLDR) and single-scattering albedo (SSA) values obtained for 1020 nm from Aerosol Robotic Network (AERONET) Version 3 Level 2.0 data collected in five regions, namely, northern China, Northwest Asia, the Tibetan Plateau, southern China and Southeast Asia, we classified seven types of aerosol (viz., purely dust, dust-dominated, pollution-dominated, non-absorbing [NA], weakly absorbing [WA], moderately absorbing [MA] and strongly absorbing [SA]) in order to assess the spatial and temporal distributions of their constituents and the radiative effects of their fine- and coarse-mode particles. The fine fraction dominated in northern China and also played a crucial role on the Tibetan Plateau and in southern OPEN ACCESS China and Southeast Asia, whereas the coarse fraction prevailed in Northwest Asia. -
Textile 9 Enterprise Name List the Name List of Enterprises in Chinese
Textile 9 Enterprise name list The Name List of Enterprises in Chinese Textile Industry Spining & Weavin g of Cotton & Chemical Fibers Dist. Name Address Zip Code Telefphone Number Core businesses Number 13 Songzhuyuan North Alley, Beiheyan Street, Beijing Snow-Lotus Woolen Dress Group 100009 010 87311909 Cotton yarn, canvas, tarpaulin Dongcheng Dist., Beijing Cotton yarn and cotton cloth Beijing Bazhen Knitting Co., Ltd. No.17, Zhongjianzi Lane, Dongcheng Dist., Beijing 100007 010 64043057 processing Beijing No.6 Knitting Co. Ltd 6, Minwang Lane, Hepingli, Dongcheng Dist., Beijing 100013 010 64280252 Cotton yarn processing Spandex yarn, blended & mixed Beijing Jingmian Textile Co. Ltd (Group) 1 Balizhuang Dongli, Chaoyang Dist., Beijing 100025 010 65584499 fabric Beijing Friendly Printing and Syeing Co., 101 Balizhuang Beili Jia, Chaoyang Dist., Beijing 100025 010 85833821 Chemical fiber manufacturing Ltd Cotton yarn/cloth and printing & Beijing Qingyun Knitting Mill 32 Nanliu Lane, Xuanwu Dist., Beijing 100052 010 13521562192 dyeing Cloth Beijing Chaoyang Dist. Jingzhan Knitting Manufacture of topgrade facsimile Xiba, Chaoyang Dist., Beijing 100018 010 84311245 Mill long/kinky fabric Beijing Rongshengda Textile Co. Ltd 1-105, 414 Building, Chaoyang Dist., Beijing 100021 010 67709239 Cotton yarn Beijing Weilijia Chemical Fibre Co. Ltd 1809 Shibalidian, Chaoyang Dist., Beijing 100023 010 67473958 Cotton yarn, cotton cloth Pure cotton yarn and mixed yarn Beijing Mengna Knitting Co., Ltd 6-101 Yanjing Xili, Chaoyang Dist., Beijing 100025 010 65938741 manufacturing Beijing Knitting Wool Factory Jia 3 No.2 Dist. , Fangxing Yuan, Fengtai Dist., Beijing 100078 010 67628881 Cotton chemical fiber processing No.190 Dawayao Village, Lugoutiao, Fengtai Dist., Meifeng Cotton Products Factory of Beijing 100071 010 83292866 Textile industry (cotton) producing Beijing Beijing Jingguan Towel Co,.