Quality of Spring Water in the Catchment Areas of the Indus River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forward Kahuta Campus
----- THE UNIVERSITY OF POONCH RAWALAKOT ----- THE UNIVERSITY OF POONCH RAWALAKOT ----- THE UNIVERSITY OF POONCH RAWALAKOT ----- --- --- THE UNIVERSITY OF POONCH RAWALAKOT AZAD JAMMU AND KASHMIR CHARTERED BY GOVERNMENT OF AJ&K AND APPROVED BY THE HIGHER EDUCATION COMMISSION ADMISSIONS FALL 2015 Forward Kahuta Campus The University of Poonch, only after 3 years of its establishment and having started 6 faculties with more than 30 programs has, launched the new campus at “Forward Kahuta” district Haveli. The new campus at Kahuta was inaugurated by the President of THE UNIVERSITY OF POONCH RAWALAKOT OF UNIVERSITY THE THE UNIVERSITY OF POONCH RAWALAKOT OF UNIVERSITY THE the State of Jammu and Kashmir and the Chancellor of the university of Poonch, Sardar Muhammad Yaqoob Khan, on June 12, 2015. At the initial stages, only two programs of 4 years English and Computer Science will be launched and ----- ----- subsequently MA/MSc., M.Phil and Ph.D. programs will be introduced. The establishment of this campus would be a great addition to the scope of the university. It would, on one hand, positively render educational services to the people of the area at their doorsteps and on the other hand, it would cater for a greater number of students, staff and Faculty which would enhance the research activities to produce quality research. Offers following 04 Years Degree Programs BS Computer Sciences BS English (Accredited by NCEAC) F.Sc. Pre-Engineering/ICS/FCS with at least 60% marks Intermediate with at least 45% marks THE UNIVERSITYTHE POONCH RAWALAKOT OF THE UNIVERSITYTHE POONCH RAWALAKOT OF HEC–NEED BASE SCHOLARSHIPS STUDENT WELFARE FUND ----- ----- HEC – Need Base Scholarships are available in the Financial assistance available by the donation of the University subject to the following conditions: different organization/individuals for the needy students 1. -

AJK at a Glance 2009
1 2 3 DEVELOPMENT SCENARIO General Azad Jammu and Kashmir lies between longitude 730 - 750 and latitude of 33o - 36o and comprises of an area of 5134 Square Miles (13297 Square Kilometers). The topography of the area is mainly hilly and mountainous with valleys and stretches of plains. Azad Kashmir is bestowed with natural beauty having thick forests, fast flowing rivers and winding streams, main rivers are Jehlum, Neelum and Poonch. The climate is sub-tropical highland type with an average yearly rainfall of 1300 mm. The elevation from sea level ranges from 360 meters in the south to 6325 meters in the north. The snow line in winter is around 1200 meters above sea level while in summer, it rises to 3300 meters. According to the 1998 population census the state of Azad Jammu & Kashmir had a population of 2.973 million, which is estimated to have grown to 3.868 million in 2009. Almost 100% population comprises of Muslims. The Rural: urban population ratio is 88:12. The population density is 291 persons per Sq. Km. Literacy rate which was 55% in 1998 census has now raised to 64%. Approximately the infant mortality rate is 56 per 1000 live births, whereas the immunization rate for the children under 5 years of age is more than 95%. The majority of the rural population depends on forestry, livestock, agriculture and non- formal employment to eke out its subsistence. Average per capita income has been estimated to be 1042 US$*. Unemployment ranges from 6.0 to 6.5%. In line with the National trends, indicators of social sector particularly health and population have not shown much proficiency. -

+44 (0) 115 911 7222 CEO MESSAGE Contents
muslimhands.org.uk | +44 (0) 115 911 7222 CEO MESSAGE Contents S U M M A R Y O F A J K P R O J E C T S 4 E D U C AT I O N & T R A I N I N G 6 I am pleased to present to you the Muslim Hands W AT E R P R O J E C T S 10 Focus Mirpur & AJK Campaign booklet. H E A LT H 14 Formally established only a year ago, the Mirpur Office has already shown encouraging results in F O O D & N U T R I T I O N 16 reaching those in need and improving lives across the district of Mirpur and beyond. E N V I R ON M E N T 18 H O W Y O U C A N H E L P 19 The tremendous success of this office is a testament not only to the dynamic team of young men and women who have worked voluntarily and relentlessly since the establishment of the office, but a credit to the generosity of the Mirpuri community in Kashmir and here in the UK. Moreover, MH Mirpur has from the outset, focused on delivering aid though strong partnerships with grass-roots community groups, the Government and the private sector. Not only does this reduce duplication and wastage – evidence shows that only such partnerships can produce lasting change. May Allah reward all those who have made this work possible. The rapidly expanding Safe Drinking Programme, which has already seen 16 modern water filtration plants installed across Mirpur in less than a year has been the most prominent intervention made my MH Mirpur – however, much good work has been done in the areas of education, medical care and environmental protection to name but a few. -

Islamic Republic of Pakistan AJK Community Development Programme – II (AJKCDP2) Final Project Design Report
Islamic Republic of Pakistan AJK Community Development Programme – II (AJKCDP2) Final project design report Main report and appendices Document Date: 2-Oct 2017 Project No. 2000001466 Report No: 4568-PK Asia and the Pacific Division Programme Management Department Islamic Republic of Pakistan AJK Community Development Programme – II (AJKCDP2) Final project design report Contents Currency equivalents iii Weights and measures iii Abbreviations and acronyms iv Map of the programme area vi Executive Summary vii Logical Framework xiv I. Strategic context and rationale 1 A. Country and rural development context 1 B. Rationale 6 II. Programme description 7 A. Programme area and target group 7 B. Development objective and impact indicators 9 C. Outcomes/Components 11 D. Lessons learned and adherence to IFAD policies and the SECAP 19 III. Programme implementation 21 A. Approach 21 B. Organizational framework 23 C. Planning, M&E, learning and knowledge management 25 D. Financial management, procurement and governance 27 E. Supervision 29 F. Risk identification and mitigation 30 IV. Progamme costs, financing, benefits and sustainability 31 A. Programme costs 31 B. Programme financing 31 C. Summary benefits and economic analysis 32 D. Sustainability 33 i Islamic Republic of Pakistan AJK Community Development Programme – II (AJKCDP2) Final project design report Appendices Appendix 1: Country and rural context background 35 Appendix 2: Poverty, targeting and gender 45 Appendix 3: Country performance and lessons learned 69 Appendix 4: Detailed programme -

According to the Syllabus of University of Azad Jammu & Kashmir
LLB FIVE YEARS DEGREE PROGRAMME NOTES According to the Syllabus of University of Azad Jammu & Kashmir Muzaffarabad and Other Public, Private Sector Universities of Pakistan Prepared By Advocate Muhammad Adnan Masood Joja Sardar Javed Zahoor Khan (Advocate) CITI Law College Rawalakot 05824-442207, 444222, 0332-4573251, 051-4852737 Near CMH Rawalakot AJK Web:-www.clc.edu.pk Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 1 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 2 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 3 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 4 Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 5 FUNCTIONAL ENGLISH Citi Law College, Near CMH, Rawalakot Ph: 05824-442207, 051-4852737 www.clc.edu.pk Page | 6 Parts of Speech NOUNS A noun is the word that refers to a person, thing or abstract idea. A noun can tell you who or what. There are several different types of noun: - There are common nouns such as dog, car, chair etc. Nouns that refer to things which can be counted (can be singular or plural) are countable nouns. Nouns that refer to some groups of countable nouns, substances, feelings and types of activity (can only be singular) are uncountable nouns. Nouns that refer to a group of people or things are collective nouns. Nouns that refer to people, organizations or places are proper nouns, only proper nouns are capitalized. -
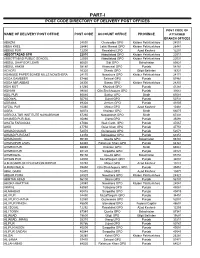
Part-I: Post Code Directory of Delivery Post Offices
PART-I POST CODE DIRECTORY OF DELIVERY POST OFFICES POST CODE OF NAME OF DELIVERY POST OFFICE POST CODE ACCOUNT OFFICE PROVINCE ATTACHED BRANCH OFFICES ABAZAI 24550 Charsadda GPO Khyber Pakhtunkhwa 24551 ABBA KHEL 28440 Lakki Marwat GPO Khyber Pakhtunkhwa 28441 ABBAS PUR 12200 Rawalakot GPO Azad Kashmir 12201 ABBOTTABAD GPO 22010 Abbottabad GPO Khyber Pakhtunkhwa 22011 ABBOTTABAD PUBLIC SCHOOL 22030 Abbottabad GPO Khyber Pakhtunkhwa 22031 ABDUL GHAFOOR LEHRI 80820 Sibi GPO Balochistan 80821 ABDUL HAKIM 58180 Khanewal GPO Punjab 58181 ACHORI 16320 Skardu GPO Gilgit Baltistan 16321 ADAMJEE PAPER BOARD MILLS NOWSHERA 24170 Nowshera GPO Khyber Pakhtunkhwa 24171 ADDA GAMBEER 57460 Sahiwal GPO Punjab 57461 ADDA MIR ABBAS 28300 Bannu GPO Khyber Pakhtunkhwa 28301 ADHI KOT 41260 Khushab GPO Punjab 41261 ADHIAN 39060 Qila Sheikhupura GPO Punjab 39061 ADIL PUR 65080 Sukkur GPO Sindh 65081 ADOWAL 50730 Gujrat GPO Punjab 50731 ADRANA 49304 Jhelum GPO Punjab 49305 AFZAL PUR 10360 Mirpur GPO Azad Kashmir 10361 AGRA 66074 Khairpur GPO Sindh 66075 AGRICULTUR INSTITUTE NAWABSHAH 67230 Nawabshah GPO Sindh 67231 AHAMED PUR SIAL 35090 Jhang GPO Punjab 35091 AHATA FAROOQIA 47066 Wah Cantt. GPO Punjab 47067 AHDI 47750 Gujar Khan GPO Punjab 47751 AHMAD NAGAR 52070 Gujranwala GPO Punjab 52071 AHMAD PUR EAST 63350 Bahawalpur GPO Punjab 63351 AHMADOON 96100 Quetta GPO Balochistan 96101 AHMADPUR LAMA 64380 Rahimyar Khan GPO Punjab 64381 AHMED PUR 66040 Khairpur GPO Sindh 66041 AHMED PUR 40120 Sargodha GPO Punjab 40121 AHMEDWAL 95150 Quetta GPO Balochistan 95151 -

Ehsaas Emergency Cash Payments
Consolidated List of Campsites and Bank Branches for Ehsaas Emergency Cash Payments Campsites Ehsaas Emergency Cash List of campsites for biometrically enabled payments in all 4 provinces including GB, AJK and Islamabad AZAD JAMMU & KASHMIR SR# District Name Tehsil Campsite 1 Bagh Bagh Boys High School Bagh 2 Bagh Bagh Boys High School Bagh 3 Bagh Bagh Boys inter college Rera Dhulli Bagh 4 Bagh Harighal BISP Tehsil Office Harigal 5 Bagh Dhirkot Boys Degree College Dhirkot 6 Bagh Dhirkot Boys Degree College Dhirkot 7 Hattain Hattian Girls Degree Collage Hattain 8 Hattain Hattian Boys High School Chakothi 9 Hattain Chakar Boys Middle School Chakar 10 Hattain Leepa Girls Degree Collage Leepa (Nakot) 11 Haveli Kahuta Boys Degree Collage Kahutta 12 Haveli Kahuta Boys Degree Collage Kahutta 13 Haveli Khurshidabad Boys Inter Collage Khurshidabad 14 Kotli Kotli Govt. Boys Post Graduate College Kotli 15 Kotli Kotli Inter Science College Gulhar 16 Kotli Kotli Govt. Girls High School No. 02 Kotli 17 Kotli Kotli Boys Pilot High School Kotli 18 Kotli Kotli Govt. Boys Middle School Tatta Pani 19 Kotli Sehnsa Govt. Girls High School Sehnsa 20 Kotli Sehnsa Govt. Boys High School Sehnsa 21 Kotli Fatehpur Thakyala Govt. Boys Degree College Fatehpur Thakyala 22 Kotli Fatehpur Thakyala Local Govt. Office 23 Kotli Charhoi Govt. Boys High School Charhoi 24 Kotli Charhoi Govt. Boys Middle School Gulpur 25 Kotli Charhoi Govt. Boys Higher Secondary School Rajdhani 26 Kotli Charhoi Govt. Boys High School Naar 27 Kotli Khuiratta Govt. Boys High School Khuiratta 28 Kotli Khuiratta Govt. Girls High School Khuiratta 29 Bhimber Bhimber Govt. -

Explore New Business in the Middle East and North the Courage and Valour to Succeed in All of Our Endeavours
Preface Environmental & Public Health Engineering Sector 28 04 Services Architecture & Planning Sector 22 03 Fields of Activity Transportation Engineering Sector 16 02 Contents List of Major New, Ongoing & Dam Engineering Sector Completed Projects 37 14 Water Resources Sector Information Technology & GIS Sector 34 10 Energy Sector Oil, Gas & Industrial Sector 32 06 Activity Fields of Water Resources Planning, Drainage, Implementation Strategies, Policy Salinity Control and Land Reclamation, Formulation, Residential Communities, Dams and Barrages, Irrigation and Urban/Rural Development Planning, Drainage Systems, Groundwater Advocacy Planning, Preparation of Resources Development, Flood Zoning Regulations and Bye-laws, Management and Forecasting/Warning Tourism Planning, Regeneration, Re- Systems and Institutional and Social settlement and Renewal Planning Development Water Supply, Sewerage, Stormwater Power Transmission and Distribution, Drainage, Solid Waste Management, Substations, Hydropower, Thermal Plumbing, Water and Wastewater Power, Nuclear Power, Rural Treatment Electrification, Supervisory Control and Data Acquisition (SCADA) and Site Characterisation/Baseline Data for Telecommunications and Oil and Gas Environmental Impact Assessment, Environmental Risk Assessment, Motorways, Highways, Urban Roads, Environmental Planning and Rural Roads, Grade-separated Management, Environmental Health and Interchanges, Bridges and Flyovers, Safety, Air and Noise Pollution Control, Subways and Underpasses, Tunnels, Contaminated Site Assessment -

Supplementary 2015
GOVERNMENT OF THE PUNJAB SUPPLEMENTARY BUDGET S T A T E M E N T For 2015-2016 I SUPPLEMENTARY BUDGET STATEMENT 2015 - 2016 SUMMARY BY DEMANDS Reference to Demand Grant Name of Demand Rs. pages Number Number I. Supplementary Demands (Voted) 1 1 3 Provincial Excise 737,724,000 2-3 2 5 Forests 21,970,000 4-9 3 9 Irrigation & Land Reclamation 2,691,013,000 10-48 4 10 General Administration 4,793,804,000 49-52 5 18 Agriculture 12,708,584,000 53 6 21 Cooperation 32,896,000 54-56 7 24 Civil Works 472,386,000 57-58 8 27 Relief 20,609,024,000 59 9 28 Pension 8,000,000,000 60-61 10 32 Civil Defence 527,676,000 62-65 11 33 State Trading in Foodgrains and Sugar 17,467,573,000 66-260 12 36 Development 67,465,788,000 261-262 13 38 Agricultural Improvement and Research 73,384,000 263-271 14 41 Roads and Bridges 6,414,569,000 272-273 15 43 Loans to Municipalities/Autonomous Bodies 4,077,214,000 Sub-Total Voted 146,093,605,000 II SUPPLEMENTARY BUDGET STATEMENT 2015 - 2016 SUMMARY BY DEMANDS Reference to Demand Grant Name of Demand Rs. pages Number Number II. Token Supplementary Demands (Voted) 274 16 1 Opium 1,000 275 17 6 Registration 1,000 276 18 7 Charges on Account of Motor Vehicles Acts 1,000 277-278 19 8 Other Taxes and Duties 1,000 279-282 20 11 Administration of Justice 1,000 283-284 21 12 Jails & Convict Settlements 1,000 285-299 22 13 Police 1,000 300 23 14 Museums 1,000 301-311 24 15 Education 1,000 312-384 25 16 Health Services 1,000 385-390 26 17 Public Health 1,000 391 27 19 Fisheries 1,000 392-398 28 20 Veterinary 1,000 399-401 29 22 Industries 1,000 402-412 30 23 Miscellaneous Departments 1,000 413-415 31 25 Communications 1,000 416 32 26 Housing & Physical Planning Department 1,000 417 33 29 Stationery and Printing 1,000 418-420 34 30 Subsidies 1,000 421-482 35 31 Miscellaneous 1,000 483-487 36 37 Irrigation Works 1,000 488-537 37 42 Government Buildings 1,000 Sub-Total Token 22,000 Total Voted 146,093,627,000 III SUPPLEMENTARY BUDGET STATEMENT 2015 - 2016 SUMMARY BY DEMANDS Reference to Demand Grant Name of Demand Rs. -

Pakistan, Second Quarter 2018
PAKISTAN, SECOND QUARTER 2018: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) - Updated 2nd edition compiled by ACCORD, 20 December 2018 Number of reported incidents with at least one fatality Number of reported fatalities National borders: GADM, November 2015a; administrative divisions: GADM, November 2015b; China/India border status: CIA, 2006; Kashmir border status: CIA, 2004; geodata of disputed borders: GADM, November 2015a; Natural Earth, undated; incident data: ACLED, 15 December 2018; coastlines and inland waters: Smith and Wessel, 1 May 2015 PAKISTAN, SECOND QUARTER 2018: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) - UPDATED 2ND EDITION COMPILED BY ACCORD, 20 DECEMBER 2018 Contents Conflict incidents by category Number of Number of reported fatalities 1 Number of Number of Category incidents with at incidents fatalities Number of reported incidents with at least one fatality 1 least one fatality Riots/protests 1325 6 9 Conflict incidents by category 2 Battles 84 53 133 Development of conflict incidents from June 2016 to June 2018 2 Violence against civilians 55 36 67 Remote violence 34 8 11 Methodology 3 Strategic developments 11 0 0 Conflict incidents per province 4 Total 1509 103 220 This table is based on data from ACLED (datasets used: ACLED, 15 December 2018). Localization of conflict incidents 4 Disclaimer 6 Development of conflict incidents from June 2016 to June 2018 This graph is based on data from ACLED (datasets used: ACLED, 15 December 2018). 2 PAKISTAN, SECOND QUARTER 2018: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) - UPDATED 2ND EDITION COMPILED BY ACCORD, 20 DECEMBER 2018 Methodology Geographic map data is primarily based on GADM, complemented with other sources if necessary. -

Azad Kashmir.Pdf
Ph: +92-51-9269519 Ph: +92-51-9269506 FEDERAL BOARD OF INTERMEDIATE E-mail:[email protected] AND SECONDARY EDUCATION Website: www.fbise.edu.pk Sector H-8/4, Islamabad, PAKISTAN No.FBISE/C-N/SSC-A/2021/464 13th April, 2021 NOTIFICATION It is hereby notified for the general information of all concerned that following Examination Centres have been established in Azad Kashmir for the conduct of SSC I & II ANNUAL EXAMINATION, 2021 of the FEDERAL BOARD OF INTERMEDIATE AND SECONDARY th EDUCATION, ISLAMABAD scheduled to be held with effect from Thursday, 20 May, 2021:- S.No Examination Centres Centre Institutions Inst Code Approx: Code Strength 1. Army Public School and 8056 a. F. G. Ashiq Hussain Public School, Bhimber (AJK) 8005 125 College, Bhimber (AJK) b. Muslim Hands Model School, Kothi Mor, Bhimber 8014 (Azad Jammu Kashmir) c. Army Public School and College, Bhimber (AJK) 8056 d. The Learners School, Bhimber, AJK 8059 e. Ex/Private candidates 2. Army Public School, P.O. and 8052 a. Army Public School,P.O and Tehsil Barnala,District Bhimber (AJK) 8052 45 Tehsil Barnala, District b. Ex/Private candidates Bhimber (AJK) 3. Army Public School, Kalri 8040 a. Army Public School, Kalri Mor, Near Ibrahimabad Bridge,Distt. 8040 74 Mor, Near Ibrahimabad Bhimber, AJK Bridge, Distt. Bhimber, AJK b. Ex/Private candidates 4. Fauji Foundation Model 8006 a. Fauji Foundation Model School, Plandri (AJK) 8006 38 School, Plandri (AJK) b. Ex/Private candidates 5. Fauji Foundation Model 8044 a. Fauji Foundation Model School, Mang (AJK) 8044 37 School, Mang (AJK) b. -
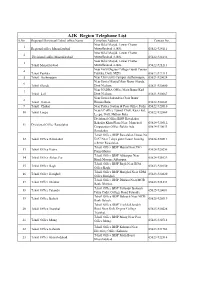
Copy of Compiled List Phone Nos BISP ALL Regions Dated 21.05
AJK Region Telephone List S.No Regioanl/Divisioanl/Tehsil office Name Complete Address Contact No. Near Bilal Masjid, Lower Chatter 1 Regional office Muzaffarabad Muzaffarabad AJ&K. 05822-924111 Near Bilal Masjid, Lower Chatter 2 Divisional office Muzaffarabad Muzaffarabad AJ&K. 05822-924132 Near Bilal Masjid, Lower Chatter 3 Tehsil Muzaffarabad Muzaffarabad AJ&K. 05822-921213 Near Girls Degree College Gandi Peeran 4 Tehsil Patikka Patikka, Distt. MZD. 05822-922113 5 Tehsil Authmaqam Near University Campus Authamaqam, 05821-920024 Near Jamia Masjid Main Bazar Sharda, 6 Tehsil Sharda Distt Neelum. 05821-920800 Near NADRA Office Main Bazar Kail 7 Tehsil kail Distt Neelum. 05821-920667 Near Jamia Sakandria Chok Bazar 8 Tehsil Hattian Hattian Bala. 05822-922643 9 Tehsil Chakar Near Police Station & Post Office Pothi 05822-922010 NearAC office Tunnel Chok, Kaser kot, 10 Tehsil Leepa 05822-922869 Leepa, Distt. Hattian Bala. Divisional Office BISP Rawalakot Bahadar Khan Plaza Near Muncipal 05824-920512, 11 Divisional Office Rawalakot Corporation Office Baldia Ada 05824-920033 Rawalakot. Tehsil Office BISP Rawalakot House No 12 Tehsil Office Rawalakot D-97 Near 7 days guest house housing 05824-920511 scheme Rawalakot. Tehsil Office BISP Hajira Near PSO 13 Tehsil Office Hajira 05824-920256 Pump Hajira. Tehsil Office BISP Abbaspur Near 14 Tehsil Office Abbas Pur 05824-921029 Hanfi Mosque Abbaspur. Tehsil Office BISP Bagh Near BDA 15 Tehsil Office Bagh 05823-920150 Office Bagh. Tehsil Office BISP Harighel Near SDM 16 Tehsil Office Harighel 05823-920820 Office Harighel. Tehsil Office BISP Dhirkot Near MCB 17 Tehsil Office Dhirkot 05823-921233 Bank Dhirkot. Tehsil Office BISP Pallandri Balouch 18 Tehsil Office Palandri 05825-920081 Palza Cadet College Road Palandri.