Immunohistochemical Characterization of Feline Lymphoplasmacytic Anterior Uveitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PG Research Day
2016 PG Research Day BOOK OF ABSTRACTS RESEARCHER ASSOCIATION PG Research Day 25th May 2016 TABLE OF CONTENTS FOREWORD ...................................................................................................................... 5 SCHEDULE FOR THE DAY .............................................................................................. 6 ORAL PRESENTATION ABSTRACTS ............................................................................. 8 Understanding anatomical movement through animations ............................................ 9 Walking with giraffes – ground reaction forces and kinematics ................................... 10 The effect of temperature rise upon immunity and susceptibility to infection in fish .... 11 Creating, testing and optimising a simulation of mouse hindlimb locomotion .............. 12 Assessing the welfare of horses in the UK ................................................................... 13 Development of a novel approach to solve genome assemblies’ jigsaw puzzles ........ 14 Investigating naïve interactions between alveolar macrophages and Mycoplasma hyopneumoniae ....................................................................................................... 15 Extracts of Hymenocardia acida Ameliorate Insulin Resistance in L6 myotubes ........ 16 Regulation of endothelial cell metabolism by PPARβ/δ and its impact on angiogenic function. ................................................................................................................... 17 Does endothelial -

Phd Student Handbook 2020/21
PhD Student Handbook 2020/21 Welcome to The Royal Veterinary College’s Graduate School Dear PhD Student The Royal Veterinary College has a unique history of innovation in biomedical and veterinary sciences, education and clinical practice. We’re delighted that you have chosen to study at the RVC and we hope that you will thoroughly enjoy your time here, acquiring the knowledge and skills you’ll need to build a successful and stimulating career. This handbook is intended to provide you with key information about your studies, the College and its many resources but if you can’t find what you’re looking for, don’t hesitate to ask one of us. We’re looking forward to working with you and wish you every success with your research. Head of the Graduate School (Academic): Prof Kristien Verheyen Email: [email protected] Ext: 6625 Head of Postgraduate Administration: Miss Natalie Hubble Email: [email protected] Ext: 6017 Postgraduate Clinical and Research Degrees Officers: Mrs Lisa Matamala-Shaw Email: [email protected] Ext: 5541 Mrs Carole Tilsley Email: [email protected] Ext: 5134 Research Admissions and Ethics Officer Emily Hicks Email: [email protected] Ext: 4612 Location: The Student Centre The Royal Veterinary College Camden Campus Website: http://www.rvc.ac.uk/study/postgraduate/graduate-school Email: [email protected] RVC Learn: https://learn.rvc.ac.uk Welcome from the RVC Postgraduate Officers Hi and welcome to the RVC! Our main role is to ensure that your voice is heard throughout the College - ranging from the SU itself to the Graduate School and other academic and non-academic forums - as well as organise events and socials for postgraduate students. -

Durham E-Theses
Durham E-Theses Non-EU International Students in UK Higher Education Institutions: Prosperity, Stagnation and Institutional Hierarchies MATEOS-GONZALEZ, JOSE,LUIS How to cite: MATEOS-GONZALEZ, JOSE,LUIS (2019) Non-EU International Students in UK Higher Education Institutions: Prosperity, Stagnation and Institutional Hierarchies, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/13359/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Non-EU International Students in UK Higher Education Institutions: Prosperity, Stagnation and Institutional Hierarchies José Luis Mateos-González Department of Sociology, Durham University A thesis submitted to Durham University for the degree of Doctor of Philosophy September 2019 1 To my mum –her unconditional support has made this thesis possible. A mi madre, cuyo apoyo incondicional ha hecho de esta tesis una realidad. To my dad –I will always miss him. -

Members of the Quality Assurance Agency for Higher Education (QAA) 2019-20
Members of the Quality Assurance Agency for Higher Education (QAA) 2019-20 The following institutions are members of QAA for 2019-20. To find out more about QAA membership, visit www.qaa.ac.uk/membership List correct at time of publication – 18 June 2020 Aberystwyth University Activate Learning AECC University College Al-Maktoum College of Higher Education Amity Global Education Limited Anglia Ruskin University Anglo American Educational Services Ltd Arden University Limited Arts University Bournemouth Ashridge Askham Bryan College Assemblies of God Incorporated Aston University Aylesbury College Bangor University Barnsley College Bath College Bath Spa University Bellerbys Educational Services Ltd (Study Group) Bexhill College Birkbeck, University of London Birmingham City University Birmingham Metropolitan College Bishop Grosseteste University Blackburn College Blackpool and The Fylde College Bolton College Bournemouth University BPP University Limited Bradford College Brockenhurst College Buckinghamshire New University Burnley College Burton & South Derbyshire College 1 Bury College Cambridge Regional College Canterbury Christ Church University Cardiff and Vale College Cardiff Metropolitan University Cardiff University CEG UFP Ltd Central Bedfordshire College Cheshire College South and West Chichester College Group Christ the Redeemer College City College Plymouth City of Bristol College City, University of London Colchester Institute Coleg Cambria Cornwall College Coventry University Cranfield University David Game College De Montfort -

Class of 2019 University Acceptances
Class of 2019 University Acceptances UK: 82% are among the TOP 50 universities in UK UK: 18 out of 25 of the prestigious universities from Russell Group UK made an offer of acceptance to one or more BFIS students US: 26% are among the TOP 50 universities in US Abroad: NYU Abu Dhabi accepted one student from BFIS who received a full scholarship worth more than $300,000 over the four years. NYU Abu Dhabi accepted 2% or 189 of the 9,048 applicants this year and is one of the most competitive schools in the world. United Kingdom Aberystwyth University Nottingham Trent University University College London Aston University Plymouth University University of Bath Bath Spa University Queen Mary, University of London University of Birmingham University of Bristol Birkbeck, University of London Royal Holloway, University of London University of East London Bristol, University of the West of England Royal Veterinary College University of Exeter City, University of London SOAS University of London University of Glasgow Durham University The University of Edinburgh University of Oxford Goldsmiths, University of London The University of Essex University of Reading Keele University The University of Liverpool University of Southampton King’s College London The University of Manchester University of Surrey University of Sussex Lancaster University The University of Nottingham University of Warwick London School of Economics and Political The University of Sheffield University of York Science The University of Stirling Loughborough University United -

College and University Acceptances
COLLEGE AND UNIVERSITY ACCEPTANCES 2016 -2020 UNITED KINGDOM • University of Bristol • High Point University • Aberystwyth University • University of Cardiff • Hofstra University • Arts University Bournemouth • University of Central Lancashire • Ithaca College • Aston University • University of East Anglia • Lesley University • Bath Spa University • University of East London • Long Island University - CW Post • Birkbeck University of London • University of Dundee • Loyola Marymount University • Bornemouth University • University of Edinburgh • Loyola University Chicago • Bristol, UWE • University of Essex • Loyola University Maryland • Brunel University London • University of Exeter • Lynn University • Cardiff University • University of Glasgow • Marist College • City and Guilds of London Art School • University of Gloucestershire • McGill University • City University of London • University of Greenwich • Miami University • Durham University • University of Kent • Michigan State University • Goldsmiths, University of London • University of Lancaster • Missouri Western State University • Greenwich School of Management • University of Leeds • New York Institute of Technology • Keele University • University of Lincoln • New York University • King’s College London • University of Liverpool • Northeastern University • Imperial College London • University of Loughborough • Oxford College of Emory University • Imperial College London - Faculty of Medicine • University of Manchester • Pennsylvania State University • International School for Screen -

Download Cardiff Exhibitor List (75.28
Stand Number Institution 17 Aberystwyth University 1 The Academy of Contemporary Music 2 AECC University College 3 Arts University Bournemouth 4 Aston University 6 Bangor University 5 University of Bath 7 Bath Spa University 8 University of Bedfordshire 9 Birmingham City University 10 University of Birmingham 11 University College Birmingham 12 Newman University 14 Bishop Grosseteste University 15 Bournemouth University 16 University of Brighton 13 The University of Bristol 18 UWE Bristol 19 Brunel University London 20 The University of Buckingham 21 Bucks New University 22 University of Cambridge 23 Canterbury Christ Church University 26 Cardiff University 24 CARDIFF METROPOLITAN UNIVERSITY 27 Cardiff and Vale College 25 Coleg Sir Gâr 28 Coleg Cymraeg Cenedlaethol 29 Coleg y Cymoedd 30 University of Chester 31 University of Chichester 32 Cornwall College 34 CU Coventry, CU London and CU Scarborough 35 Coventry University 38 University for the Creative Arts 33 De Montfort University 36 University of Dundee 37 Durham University 39 University of East Anglia (UEA) 40 Echo Factory 41 Edge Hill University 42 The University of Edinburgh 43 University of Essex 44 University of Exeter 45 Falmouth University 46 The Glasgow School of Art 47 University of Gloucestershire 48 Wrexham Glyndwr University 49 Harper Adams University 50 Hereford College of Arts 51 Hartpury University 52 Heriot-Watt University 53 University of the Highlands and Islands 54 University of Huddersfield 55 University of Hull 56 IE University, Spain 58 Imperial College London -

British School of Brussels Uk Universities Fair 2020
BRITISH SCHOOL OF BRUSSELS UK UNIVERSITIES FAIR 2020 20th October Times in Brussels Time 17.30 – 18.00 18.15 – 18.45 19.00 – 19.30 19.45 – 20.15 20.30 – 21.00 Your Future in Tech Royal Veterinary College Information University of Westminster Information Abertay University Information Session (Abertay University) Abertay University Information Session Session Session Applying for Competitive Courses in the UK Studying Engineering in the UK University of Bristol Information Session Engineering opportunities at the University (University of Bath) of Bristol University of Bristol Information Session (Imperial College London) UK-US Joint Degree: The University of Understanding the Multiple Mini Interview Andrews BA International Honours (MMI) Imperial College London Information Imperial College London Information programme University of St Andrews Information Royal Veterinary College Session Session (University of St Andrews) Session University of St Andrews Information Royal Veterinary College Information London School of Economics Information London School of Economics Information Arts University Bournemouth Information Session Session Session Session Session Falmouth University Information Session Falmouth University Information Session University of Sussex Information Session University of Sussex Information Session Newcastle University Information Session Choosing a degree to study in the UK Arts University Bournemouth Information Choosing the right fit UK university (University of Leeds) University of Leeds Information Session Session -
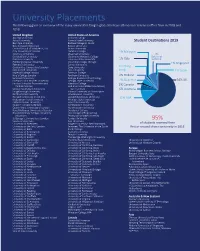
University Placements the Following Gives an Overview of the Many Universities Tanglin Graduates Have Attended Or Received Offers from in 2018 and 2019
University Placements The following gives an overview of the many universities Tanglin graduates have attended or received offers from in 2018 and 2019. United Kingdom United States of America Abertay University Amherst College Aston University Arizona State University Student Destinations 2019 Bath Spa University Berklee College of Music Bournemouth University Boston University Central School of St Martin’s, UAL Brown University City, University of London Carleton College 1% Malaysia Coventry University Chapman University 7% De Montfort University Claremont McKenna College National Durham University Colorado State University 1% Italy Service Edinburgh Napier University Columbia College, Chicago Falmouth University Cornell University 1% Singapore Goldsmiths, University of London Duke University 1% Hong Heriot-Watt University Elon University Kong 1% Spain Imperial College London Emerson College King’s College London Fordham University 2% Holland Lancaster University Georgia Institute of Technology Liverpool John Moores University Georgia State University 4% Gap Year 64% UK London School of Economics and Hamilton College Political Science Hult International Business School, 2% Canada London South Bank University San Francisco 6% Australia Loughborough University Indiana University at Bloomington Northumbria University John Hopkins University Norwich University of the Arts Loyola Marymount University 11% USA Nottingham Trent University Michigan State University Oxford Brookes University New York University Queen’s University Belfast Northeastern -

Giardia Secretome Highlights Secreted Tenascins As a Key Component of Pathogenesis
Title: Giardia Secretome Highlights Secreted Tenascins as a Key Component of Pathogenesis Running Title: Giardia Secretome Authors: Audrey Dubourga, Dong Xiab,e, John P. Winpennya, Suha Al Naimia,c, Maha Bouzida, Darren W. Sextona,d, Jonathan M. Wastlingb,f, Paul R. Huntera and Kevin M. Tyler*a Authors’ affiliations: a. NIHR Health Protection Research Unit in Gastrointestinal Infections, Norwich Medical School, University of East Anglia, Norwich, UK. b. Department of Infection Biology, Institute of Infection and Global Health, Faculty of Health & Life Sciences, University of Liverpool, UK. c. Department of Science and Technology, Faculty of Health and Science, University of Suffolk, Ipswich, UK d. School of Pharmacy and Biomolecular Sciences Liverpool John Moores University, Liverpool, UK. e. Research Support Office, Royal Veterinary College, University of London, UK. f. Faculty of Natural Sciences, Keele University, Staffordshire, UK. Authors email: AD: [email protected] DX: [email protected] JPW: [email protected] SAN: [email protected] Downloaded from https://academic.oup.com/gigascience/advance-article-abstract/doi/10.1093/gigascience/giy003/4818238 by Keele University user on 02 February 2018 MB: [email protected] DWS: [email protected] JMW: [email protected] PRH: [email protected] KT: [email protected] *Correspondence: [email protected], Tel +44 (0)1603-591225, Fax: +44 (0) 1603 591750. Abstract Background: Giardia is a protozoan parasite of public health relevance that causes gastroenteritis in a wide range of hosts. Two genetically distinct lineages (assemblages A and B) are responsible for the human disease. -

Veterinary Students As Global Citizens
Veterinary Students as Global Citizens Exploring opportunities for embedding the global dimension in the undergraduate veterinary curriculum Funded by This report has been funded by UK aid from the UK Government, however the views expressed do not necessarily reflect the UK Government’s official policies. The views expressed in this report are the opinions of the authors based on the experience gained during the Students as Global Citizens project and do not necessarily reflect the views of their institutions or wider professions. 1 Veterinary Students as Global Citizens Exploring opportunities for embedding the global dimension in the undergraduate veterinary curriculum Authors Jenny Maud Project Officer, Students as Global Citizens Royal Veterinary College Nicole Blum Lecturer in Development Education Development Education Research Centre Institute of Education Nick Short Head, E-Media Unit Royal Veterinary College Nigel Goode Senior Lecturer in Comparative Biomedical Sciences Royal Veterinary College © Royal Veterinary College, University of London and Development Education Research Centre, Institute of Education, 2012. Published by Royal Veterinary College and Development Education Research Centre, Institute of Education. ISBN: 978-0-9574546-4-4 2 Contents Foreword 3 Executive Summary 4 Introduction 5 What is the global dimension of veterinary medicine? 8 Why is it important to include the global dimension in veterinary education? 12 How can the global dimension be included in the veterinary curriculum? 16 What are the key challenges? 24 Conclusions 28 Further resources on global veterinary medicine and global awareness 29 References 30 3 Foreword Veterinary surgeons are extremely well placed to positively influence the future health and welfare of all animals, including humans. -

Here Possible to Prioritise Learning and Student Experience
London Higher Task Group: Safe Return to Work & Study COVID-19 Secure Charter 1. London HEIs are working to ensure that our campus estates across the capital are COVID- 19 Secure for staff and students, following current government guidance, legal requirements and recommendations from UUK. For example, The University of Westminster have established a task force - the Being Safe, Feeling Safe team - to lead on planning and make preparations for the year ahead. The University of London Facilities Management Directorate has developed strategies and tools to facilitate the safe return to buildings, which include post lockdown re-entry strategy and organisational safety recovery risk assessments. UCL have been running pilot schemes in a limited number of buildings to see how staff and students can work in a safe and socially distanced environment, and will use the learning from these schemes in a phased and measured return to campus with a wide range of safety measures in place. 2. London HEIs are working to ensure that social distancing will be maintained, as reasonably practicable, throughout campus estates and are actively managing numbers of staff and students on campus. London’s HEIs will offer a combination of online and face-to-face solutions to working and learning to ensure that social distancing can be maintained in all buildings. We are committed to finding innovative uses of university estates and repurposing space where possible to prioritise learning and student experience. All universities are providing appropriate signage to assist with social distancing and directional access and progress through buildings as well as socially distanced room layouts and hand sanitisation stations.