Diversity of Baculoviruses Isolated from Cutworms (Agrotis Spp.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entomofauna Ansfelden/Austria; Download Unter
©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 28: 377-388 ISSN 0250-4413 Ansfelden, 30. November 2007 Phytophagous Noctuidae (Lepidoptera) of the Western Black Sea Region and their ichneumonid parasitoids Z. OKYAR & M. YURTCAN Abstract Eleven agricultural and silviculturally important species of Noctuidae and their parasitoids were determined in 33 localities from the Western Black Sea region between 2001 and 2004. The ichneumonid biological control agents Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans were obtained by rearing the host larvae. K e y w o r d s : Lepidoptera, Noctuidae, Hymenoptera, Ichneumonidae, parasitoidism, Western Black Sea Region, Turkey Zusammenfassung 11 land- und forstwirtschaftlich bedeutende Noctuidae-Arten einschließlich ihrer Parasitoide aus 33 Standorten des Gebietes des westlichen Schwarzen Meeres wurden im Zeitraum 2001 bis 2004 studiert. Ichneumonidae der Arten Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans konnten durch Aufzucht der Wirtslarven festgestellt werden. 377 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Introduction The Noctuidae is the largest family of the Lepidoptera. Larvae of some species are par- ticularly harmful to agricultural and silvicultural regions worldwide. Consequently, for years intense efforts have been carried out to control them through chemical, biological, and cultural methods (LIBURD et al. 2000; HOBALLAH et al. 2004; TOPRAK & GÜRKAN 2005). In the field, noctuid control is often carried out by parasitoid wasps (CHO et al. 2006). Ichneumonids are one of the most prevalent parasitoid groups of noctuids but they also parasitize on other many Lepidoptera, Coleoptera, Hymenoptera, Diptera and Araneae (KASPARYAN 1981; FITTON et al. 1987, 1988; GAULD & BOLTON 1988; WAHL 1993; GEORGIEV & KOLAROV 1999). -

Protein Composition of the Occlusion Bodies of Epinotia Aporema Granulovirus
bioRxiv preprint doi: https://doi.org/10.1101/465021; this version posted November 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Protein composition of the occlusion bodies of Epinotia aporema 2 granulovirus 3 4 Tomás Masson, María Laura Fabre, María Leticia Ferrelli, Matías Luis Pidre, Víctor 5 Romanowski. 6 1 Instituto de Biotecnología y Biología Molecular (IBBM, UNLP-CONICET), Facultad de 7 Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina. 8 9 Abstract 10 Within family Baculoviridae, members of the Betabaculovirus genus are employed as 11 biocontrol agents against lepidopteran pests, either alone or in combination with 12 selected members of the Alphabaculovirus genus. Epinotia aporema granulovirus 13 (EpapGV) is a fast killing betabaculovirus that infects the bean shoot borer (E. 14 aporema) and is a promising biopesticide. Because occlusion bodies (OBs) play a key 15 role in baculovirus horizontal transmission, we investigated the composition of 16 EpapGV OBs. Using mass spectrometry-based proteomics we could identify 56 proteins 17 that are included in the OBs during the final stages of larval infection. Our data 18 provides experimental validation of several annotated hypothetical coding sequences. 19 Proteogenomic mapping against genomic sequence detected a previously unannotated 20 ac110-like core gene and a putative translation fusion product of ORFs epap48 and 21 epap49. Comparative studies of the proteomes available for the family Baculoviridae 22 highlight the conservation of core gene products as parts of the occluded virion. -

Ewsletter Before May 15
EWSLET TER of the MICHIGAN ENTOMOLOGICAL SOCIETY 2&3 April 25, 1986 T h i r t y - sec 0 n d An nua I M e e tin 9 P I ann e d Mark O'Brien has prepared an excellent ~ session for this year's annual meeting at the ~ University of Michigan's Matthaei Botanical ~ Gardens. Our featured speaker will be Robert Matthews from the University of Georgia. The extra symposium topic planned this year focu (-----: ses on the Great Lakes Region fauna. There will be a lobby display on "Michigan Insects." Mark chose Zingerman's Deli for the catered luncheon. He says they always have a superior spread. As an added feature, , several companies have donated products which r-· -~--, we will give away as door prizes. And don't : ,----....... forget the poster contest (rules in last ,-------, news let ter). Mark has gone all out for this meeting, {' so how about joining us! There will be the usual evening collecting, and of course, --' tours of the fabulous gardens and greenhouses. Put the date on your calendar now and send in the pre-registration form from this newsletter before May 15. See you there! 32nd Annual Meeting Michigan Entomological Society June 6, 1986 co.. 'Iz. ti\i. Matthaei Botanical Gardens 1800 Dixboro Road Ann Arbor, MICH 48105 Call: Mark O'Brien at (313) 764-0471 for further information. The NEWSLETTER of the Michigan Entomological Society is published as four numbers yearly, at irregular intervals. Please send all notes, news, new insect records, research requests, notices, season summaries, membership inquiries, etc. -

Tically Expands Our Understanding on Virosphere in Temperate Forest Ecosystems
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 June 2021 doi:10.20944/preprints202106.0526.v1 Review Towards the forest virome: next-generation-sequencing dras- tically expands our understanding on virosphere in temperate forest ecosystems Artemis Rumbou 1,*, Eeva J. Vainio 2 and Carmen Büttner 1 1 Faculty of Life Sciences, Albrecht Daniel Thaer-Institute of Agricultural and Horticultural Sciences, Humboldt-Universität zu Berlin, Ber- lin, Germany; [email protected], [email protected] 2 Natural Resources Institute Finland, Latokartanonkaari 9, 00790, Helsinki, Finland; [email protected] * Correspondence: [email protected] Abstract: Forest health is dependent on the variability of microorganisms interacting with the host tree/holobiont. Symbiotic mi- crobiota and pathogens engage in a permanent interplay, which influences the host. Thanks to the development of NGS technol- ogies, a vast amount of genetic information on the virosphere of temperate forests has been gained the last seven years. To estimate the qualitative/quantitative impact of NGS in forest virology, we have summarized viruses affecting major tree/shrub species and their fungal associates, including fungal plant pathogens, mutualists and saprotrophs. The contribution of NGS methods is ex- tremely significant for forest virology. Reviewed data about viral presence in holobionts, allowed us to address the role of the virome in the holobionts. Genetic variation is a crucial aspect in hologenome, significantly reinforced by horizontal gene transfer among all interacting actors. Through virus-virus interplays synergistic or antagonistic relations may evolve, which may drasti- cally affect the health of the holobiont. Novel insights of these interplays may allow practical applications for forest plant protec- tion based on endophytes and mycovirus biocontrol agents. -

Moths of Ohio Guide
MOTHS OF OHIO field guide DIVISION OF WILDLIFE This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any unauthorized INTRODUCTION reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wildlife and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. Text by: David J. Horn Ph.D Moths are one of the most diverse and plentiful HOW TO USE THIS GUIDE groups of insects in Ohio, and the world. An es- Scientific Name timated 160,000 species have thus far been cata- Common Name Group and Family Description: Featured Species logued worldwide, and about 13,000 species have Secondary images 1 Primary Image been found in North America north of Mexico. Secondary images 2 Occurrence We do not yet have a clear picture of the total Size: when at rest number of moth species in Ohio, as new species Visual Index Ohio Distribution are still added annually, but the number of species Current Page Description: Habitat & Host Plant is certainly over 3,000. Although not as popular Credit & Copyright as butterflies, moths are far more numerous than their better known kin. There is at least twenty Compared to many groups of animals, our knowledge of moth distribution is very times the number of species of moths in Ohio as incomplete. Many areas of the state have not been thoroughly surveyed and in some there are butterflies. counties hardly any species have been documented. Accordingly, the distribution maps in this booklet have three levels of shading: 1. -

Expression, Delivery and Function of Insecticidal Proteins Expressed by Recombinant Baculoviruses
Viruses 2015, 7, 422-455; doi:10.3390/v7010422 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Expression, Delivery and Function of Insecticidal Proteins Expressed by Recombinant Baculoviruses Jeremy A. Kroemer 1,2, Bryony C. Bonning 1 and Robert L. Harrison 3,* 1 Department of Entomology, Iowa State University, Ames, IA 50011, USA; E-Mails: [email protected] (J.A.K.); [email protected] (B.C.B.) 2 Current location and contact information: Monsanto Company, 700 Chesterfield Parkway West, Chesterfield, MO 63017, USA 3 USDA-ARS Beltsville Agricultural Research Center, Invasive Insect Biocontrol & Behavior Laboratory, 10300 Baltimore Ave, Beltsville, MD 20705, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-301-504-5249; Fax: +1-301-504-5104. Academic Editor: John Burand and Madoka Nakai Received: 25 November 2014 / Accepted: 15 January 2015 / Published: 21 January 2015 Abstract: Since the development of methods for inserting and expressing genes in baculoviruses, a line of research has focused on developing recombinant baculoviruses that express insecticidal peptides and proteins. These recombinant viruses have been engineered with the goal of improving their pesticidal potential by shortening the time required for infection to kill or incapacitate insect pests and reducing the quantity of crop damage as a consequence. A wide variety of neurotoxic peptides, proteins that regulate insect physiology, degradative enzymes, and other potentially insecticidal proteins have been evaluated for their capacity to reduce the survival time of baculovirus-infected lepidopteran host larvae. Researchers have investigated the factors involved in the efficient expression and delivery of baculovirus-encoded insecticidal peptides and proteins, with much effort dedicated to identifying ideal promoters for driving transcription and signal peptides that mediate secretion of the expressed target protein. -

Diversity of Large DNA Viruses of Invertebrates ⇑ Trevor Williams A, Max Bergoin B, Monique M
Journal of Invertebrate Pathology 147 (2017) 4–22 Contents lists available at ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip Diversity of large DNA viruses of invertebrates ⇑ Trevor Williams a, Max Bergoin b, Monique M. van Oers c, a Instituto de Ecología AC, Xalapa, Veracruz 91070, Mexico b Laboratoire de Pathologie Comparée, Faculté des Sciences, Université Montpellier, Place Eugène Bataillon, 34095 Montpellier, France c Laboratory of Virology, Wageningen University, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands article info abstract Article history: In this review we provide an overview of the diversity of large DNA viruses known to be pathogenic for Received 22 June 2016 invertebrates. We present their taxonomical classification and describe the evolutionary relationships Revised 3 August 2016 among various groups of invertebrate-infecting viruses. We also indicate the relationships of the Accepted 4 August 2016 invertebrate viruses to viruses infecting mammals or other vertebrates. The shared characteristics of Available online 31 August 2016 the viruses within the various families are described, including the structure of the virus particle, genome properties, and gene expression strategies. Finally, we explain the transmission and mode of infection of Keywords: the most important viruses in these families and indicate, which orders of invertebrates are susceptible to Entomopoxvirus these pathogens. Iridovirus Ó Ascovirus 2016 Elsevier Inc. All rights reserved. Nudivirus Hytrosavirus Filamentous viruses of hymenopterans Mollusk-infecting herpesviruses 1. Introduction in the cytoplasm. This group comprises viruses in the families Poxviridae (subfamily Entomopoxvirinae) and Iridoviridae. The Invertebrate DNA viruses span several virus families, some of viruses in the family Ascoviridae are also discussed as part of which also include members that infect vertebrates, whereas other this group as their replication starts in the nucleus, which families are restricted to invertebrates. -

PRODUCTION of CYDIA POMONELLA GRANULOVIRUS (Cpgv) in a HETERALOGOUS HOST, THAUMATOTIBIA LEUCOTRETA (MEYRICK) (FALSE CODLING MOTH)
PRODUCTION OF CYDIA POMONELLA GRANULOVIRUS (CpGV) IN A HETERALOGOUS HOST, THAUMATOTIBIA LEUCOTRETA (MEYRICK) (FALSE CODLING MOTH). A thesis submitted in fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY of RHODES UNIVERSITY By CRAIG BRIAN CHAMBERS December 2014 ABSTRACT Cydia pomonella (Linnaeus) (Family: Tortricidae), the codling moth, is considered one of the most significant pests of apples and pears worldwide, causing up to 80% crop loss in orchards if no control measures are applied. Cydia pomonella is oligophagous feeding on a number of alternate hosts including quince, walnuts, apricots, peaches, plums and nectarines. Historically the control of this pest has been achieved with the use of various chemical control strategies which have maintained pest levels below the economic threshold at a relatively low cost to the grower. However, there are serious concerns surrounding the use of chemical insecticides including the development of resistance in insect populations, the banning of various insecticides, regulations for lowering of the maximum residue level and employee and consumer safety. For this reason, alternate measures of control are slowly being adopted by growers such as mating disruption, cultural methods and the use of baculovirus biopesticides as part of integrated pest management programmes. The reluctance of growers to accept baculovirus or other biological control products in the past has been due to questionable product quality and inconsistencies in their field performance. Moreover, the development and application of biological control products is more costly than the use of chemical alternatives. Baculoviruses are arthropod specific viruses that are highly virulent to a number of lepidopteran species. Due to the virulence and host specificity of baculoviruses, Cydia pomonella granulovirus has been extensively and successfully used as part of integrated pest management systems for the control of C. -

Biological Characteristics of Experimental Genotype Mixtures of Cydia Pomonella Granulovirus
Biological characteristics of experimental genotype mixtures of Cydia pomonella Granulovirus (CpGV): Ability to control susceptible and resistant pest populations Benoit Graillot, Sandrine Bayle, Christine Blachère-Lopez, Samantha Besse, Myriam Siegwart, Miguel Lopez-Ferber To cite this version: Benoit Graillot, Sandrine Bayle, Christine Blachère-Lopez, Samantha Besse, Myriam Siegwart, et al.. Biological characteristics of experimental genotype mixtures of Cydia pomonella Granulovirus (CpGV): Ability to control susceptible and resistant pest populations. Viruses, MDPI, 2016, 8 (5), 12 p. 10.3390/v8050147. hal-01594837 HAL Id: hal-01594837 https://hal.archives-ouvertes.fr/hal-01594837 Submitted on 20 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License viruses Article Biological Characteristics of Experimental Genotype Mixtures of Cydia Pomonella Granulovirus (CpGV): Ability to Control Susceptible and Resistant Pest Populations Benoit Graillot 1,2, Sandrine Bayle 1, Christine -

EXTERNAL GENITALIC MORPHOLOGY and COPULATORY MECHANISM of CYANOTRICHA NECYRIA (FELDER) (DIOPTIDAE) Genitalic Structure Has Been
Journal of the Lepidopterists' Society 42(2). 1988, 103-115 EXTERNAL GENITALIC MORPHOLOGY AND COPULATORY MECHANISM OF CYANOTRICHA NECYRIA (FELDER) (DIOPTIDAE) JAMES S. MILLER Curatorial Fellow, Department of Entomology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024 ABSTRACT. External genitalia of Cyanotricha necyria (Felder) exhibit characters that occur in the Notodontidae and Dioptidae. These provide further evidence that the two groups are closely related. Dissection of two C. necyria pairs in copula revealed two features unique among copulatory mechanisms described in Lepidoptera. First, only the male vesica, rather than the aedoeagus and vesica, are inserted into the female. Secondly, during copulation the female is pulled into the male abdomen, and his eighth segment applies dorsoventral pressure on the female's seventh abdominal segment. This mechanism is facilitated by a long membrane between the male eighth and ninth abdominal segments. The first trait is probably restricted to only some dioptid species, while the second may represent a synapomorphy for a larger group that would include all dioptids, and all or some notodontids. Additional key words: Noctuoidea, Notodontidae, Josiinae, functional morphology. Genitalic structure has been one of the most important sources of character information in Lepidoptera systematics. Taxonomists often use differences in genitalic morphology to separate species, and ho mologous similarities have provided characters for defining higher cat egories in Lepidoptera classification (Mehta 1933, Mutuura 1972, Dug dale 1974, Common 1975). Unfortunately, we know little concerning functional morphology of genitalia. A knowledge of function may aid in determining homology of genitalic structures, something that has proved to be extremely difficult and controversial. -
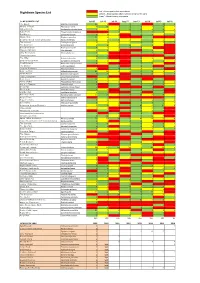
Highdown Species List 2008 and 2009 and 2010 and 2011 and 2012 and 2013 and 2014 and 2015 and 2016.Xlsx
'red' = those species that were absent Highdown Species List 'yellow' = those species who's numbers remained the same 'green' = those showing an increase CORE SURVEY LIST Jul‐09 Jul‐10 Jul‐11 Aug‐12 Jun‐13 Jul‐14 Jul‐15 Jul‐16 The Snout Hypena proboscidalis 1142322 Mother of Pearl Pleuroptya ruralis 911334242 Willow Beauty Peribatodes rhomboidaria 76368491 Ruby Tiger Phragmatobia fuliginosa 212232 Buff Ermine Spilosoma luteum 862 2913 Muslin Moth Diaphora mendica 12 6 2 1 Broad-bordered Yellow Underwing Noctua fimbriata 52621311 The Coronet Craniophora ligustri 43114725 The Sycamore Acronicta aceris 2323232 Burnished Brass Diachrysia chrysitis 632332 Chinese Character Cilix glaucata 131 21 Grey Pine Carpet Thera obeliscata 74 333 Carcina quercana 23 1 The Miller Acronicta leporine 68 642 Swallow-tailed Moth Ourapteryx sambucaria 951 6243 Small Emerald Hemistola chrysoprasaria 81 232 The Drinker Euthrix potatoria 1122 Swallow Prominent Pheosia tremula 4 34212 Rosy Footman Miltochrista miniata 4118224946 White Ermine Spilosoma lubricipeda 791 3623 True Lover's Knot Lycophotia porphyrea 1 Knot Grass Acronicta rumicis 127352 Angle Shades Phlogophora meticulosa 84114121 Nut-tree Tussock Colocasia coryli 4 4 3341 Brown-tail Euproctis chrysorrhoea 72 24 Blood-vein Timandra comae 72 1112 Slender Pug Eupithecia tenuiata 24 5 1 Brimstone Moth Opisthograptis luteolata 11 19 2 7 12 6 6 4 Purple Thorn Selenia tetralunaria 1 2 Peppered Moth Biston betularia 75126253 Buff Arches Habrosyne pyritoides 532 4464 Setaceous Hebrew Character Xestia c‐nigrum -

Baculovirus Enhancins and Their Role in Viral Pathogenicity
9 Baculovirus Enhancins and Their Role in Viral Pathogenicity James M. Slavicek USDA Forest Service USA 1. Introduction Baculoviruses are a large group of viruses pathogenic to arthropods, primarily insects from the order Lepidoptera and also insects in the orders Hymenoptera and Diptera (Moscardi 1999; Herniou & Jehle, 2007). Baculoviruses have been used to control insect pests on agricultural crops and forests around the world (Moscardi, 1999; Szewczk et al., 2006, 2009; Erlandson 2008). Efforts have been ongoing for the last two decades to develop strains of baculoviruses with greater potency or other attributes to decrease the cost of their use through a lower cost of production or application. Early efforts focused on the insertion of foreign genes into the genomes of baculoviruses that would increase viral killing speed for use to control agricultural insect pests (Black et al., 1997; Bonning & Hammock, 1996). More recently, research efforts have focused on viral genes that are involved in the initial and early processes of infection and host factors that impede successful infection (Rohrmann, 2011). The enhancins are proteins produced by some baculoviruses that are involved in one of the earliest events of host infection. This article provides a review of baculovirus enhancins and their role in the earliest phases of viral infection. 2. Lepidopteran specific baculoviruses The Baculoviridae are divided into four genera: the Alphabaculovirus (lepidopteran-specific nucleopolyhedroviruses, NPV), Betabaculovirus (lepidopteran specific Granuloviruses, GV), Gammabaculovirus (hymenopteran-specific NPV), and Deltabaculovirus (dipteran-specific NPV) (Jehle et al., 2006). Baculoviruses are arthropod-specific viruses with rod-shaped nucleocapsids ranging in size from 30-60 nm x 250-300 nm.