Esophageal Strictures and Diverticula
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Candida Esophagitis Complicated by Esophageal Stricture
E180 UCTN – Unusual cases and technical notes Candida esophagitis complicated by esophageal stricture Fig. 1 Esophageal luminal narrowing was Fig. 2 Follow-up endoscopy performed Fig. 3 Follow-up endoscopy for the evalua- observed at 23 cm from the central incisor 6 weeks after the initial evaluation at our hos- tion of dysphagia 3 months after the initiation with irregular mucosa and multiple whitish pital showed improvement of inflammation, of treatment with a antifungal agent revealed exudates, through which the scope (GIF-H260, but still the narrowed lumen did not allow the further stenosed lumen, through which not Olympus, Japan) could not pass. passage of the endoscope. even the GIF-Q260, an endoscope of smaller caliber than the GIF-H260, could pass. A 31-year-old woman was referred to the department of gastroenterology with dys- phagia accompanied by odynophagia without weight loss. The patient was immunocompetent and her only medica- tion was synthyroid, which she had been taking for the past 15 years due to hypo- thyroidism. The patient said that she had her first recurrent episodes of odynopha- gia 7 years previously and recalled that endoscopic examination at that time had revealed severe candida esophagitis. Her symptoms improved after taking medi- cation for 1 month. She was without symptoms for a couple of years, but about 5 years prior to the current presentation, Fig. 4 Barium esophagogram demonstrated narrowing of the upper and mid-esophagus (arrows) she began to experience dysphagia from with unaffected distal esophagus. time to time when taking pills or swal- lowing meat, and these episodes had be- come more frequent and had worsened cer and pseudoepitheliomatous hyperpla- the GIF-Q260 could not pass (●" Fig. -

Dysphagia - Pathophysiology of Swallowing Dysfunction, Symptoms, Diagnosis and Treatment
ISSN: 2572-4193 Philipsen. J Otolaryngol Rhinol 2019, 5:063 DOI: 10.23937/2572-4193.1510063 Volume 5 | Issue 3 Journal of Open Access Otolaryngology and Rhinology REVIEW ARTICLE Dysphagia - Pathophysiology of Swallowing Dysfunction, Symptoms, Diagnosis and Treatment * Bahareh Bakhshaie Philipsen Check for updates Department of Otorhinolaryngology-Head and Neck Surgery, Odense University Hospital, Denmark *Corresponding author: Dr. Bahareh Bakhshaie Philipsen, Department of Otorhinolaryngology-Head and Neck Surgery, Odense University Hospital, Sdr. Boulevard 29, 5000 Odense C, Denmark, Tel: +45 31329298, Fax: +45 66192615 the vocal folds adduct to prevent aspiration. The esoph- Abstract ageal phase is completely involuntary and consists of Difficulty swallowing is called dysphagia. There is a wide peristaltic waves [2]. range of potential causes of dysphagia. Because there are many reasons why dysphagia can occur, treatment Dysphagia is classified into the following major depends on the underlying cause. Thorough examination types: is important, and implementation of a treatment strategy should be based on evaluation by a multidisciplinary team. 1. Oropharyngeal dysphagia In this article, we will describe the mechanism of swallowing, the pathophysiology of swallowing dysfunction and different 2. Esophageal dysphagia causes of dysphagia, along with signs and symptoms asso- 3. Complex neuromuscular disorders ciated with dysphagia, diagnosis, and potential treatments. 4. Functional dysphagia Keywords Pathophysiology Dysphagia, Deglutition, Deglutition disorders, FEES, Video- fluoroscopy Swallowing is a complex process and many distur- bances in oropharyngeal and esophageal physiology including neurologic deficits, obstruction, fibrosis, struc- Introduction tural damage or congenital and developmental condi- Dysphagia is derived from the Greek phagein, means tions can result in dysphagia. Breathing difficulties can “to eat” [1]. -

From Inflammatory Bowel Diseases to Endoscopic Surgery Kentaro Iwata1,2†, Yohei Mikami1*† , Motohiko Kato1,2, Naohisa Yahagi2 and Takanori Kanai1*
Iwata et al. Inflammation and Regeneration (2021) 41:21 Inflammation and Regeneration https://doi.org/10.1186/s41232-021-00174-7 REVIEW Open Access Pathogenesis and management of gastrointestinal inflammation and fibrosis: from inflammatory bowel diseases to endoscopic surgery Kentaro Iwata1,2†, Yohei Mikami1*† , Motohiko Kato1,2, Naohisa Yahagi2 and Takanori Kanai1* Abstract Gastrointestinal fibrosis is a state of accumulated biological entropy caused by a dysregulated tissue repair response. Acute or chronic inflammation in the gastrointestinal tract, including inflammatory bowel disease, particularly Crohn’s disease, induces fibrosis and strictures, which often require surgical or endoscopic intervention. Recent technical advances in endoscopic surgical techniques raise the possibility of gastrointestinal stricture after an extended resection. Compared to recent progress in controlling inflammation, our understanding of the pathogenesis of gastrointestinal fibrosis is limited, which requires the development of prevention and treatment strategies. Here, we focus on gastrointestinal fibrosis in Crohn’s disease and post-endoscopic submucosal dissection (ESD) stricture, and we review the relevant literature. Keywords: Gastrointestinal fibrosis, Crohn’s disease, Endoscopic surgery Background surgical wounds. Fibrostenosis of the gastrointestinal Gastrointestinal stricture is the pathological thickening tract, in particular, is a frequent complication of Crohn’s of the wall of the gastrointestinal tract, characterized by disease. Further, a recent highly significant advance in excessive accumulation of extracellular matrix (ECM) endoscopic treatment enables resection of premalignant and expansion of the population of mesenchymal cells. and early-stage gastrointestinal cancers. This procedure Gastrointestinal stricture leads to blockage of the gastro- does not involve surgical reconstruction of the gastro- intestinal tract, which significantly reduces a patient’s intestinal tract, although fibrotic stricture after endo- quality of life. -
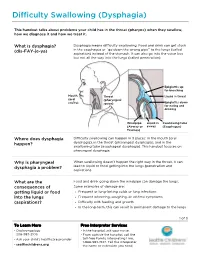
PE3334 Difficulty Swallowing (Dysphagia)
Difficulty Swallowing (Dysphagia) This handout talks about problems your child has in the throat (pharynx) when they swallow, how we diagnose it and how we treat it. What is dysphagia? Dysphagia means difficulty swallowing. Food and drink can get stuck (dis-FAY-je-ya) in the esophagus or “go down the wrong pipe” to the lungs (called aspiration) instead of the stomach. It can also go into the voice box but not all the way into the lungs (called penetration). Epiglottis up for breathing Mouth Throat Liquid in throat (oral (pharyngeal cavity) space) Epiglottis down for eating and drinking Windpipe Liquid in Swallowing tube (Airway or airway (Esophagus) Trachea) Where does dysphagia Difficulty swallowing can happen in 3 places: in the mouth (oral happen? dysphagia), in the throat (pharyngeal dysphagia), and in the swallowing tube (esophageal dysphagia). This handout focuses on pharyngeal dysphagia. Why is pharyngeal When swallowing doesn’t happen the right way in the throat, it can dysphagia a problem? lead to liquid or food getting into the lungs (penetration and aspiration). What are the Food and drink going down the windpipe can damage the lungs. consequences of Some examples of damage are: getting liquid or food • Frequent or long-lasting colds or lung infections into the lungs • Frequent wheezing, coughing, or asthma symptoms (aspiration)? • Difficulty with feeding and growth • In the long-term, this can result in permanent damage to the lungs 1 of 3 To Learn More Free Interpreter Services • Otolaryngology • In the hospital, ask your nurse. 206-987-2105 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. -

The Gastrointestinal System and the Elderly
2 The Gastrointestinal System and the Elderly Thomas W. Sheehy 2.1. Introduction Gastrointestinal diseases increase with age, and their clinical presenta tions are often confused by functional complaints and by pathophysio logic changes affecting the individual organs and the nervous system of the gastrointestinal tract. Hence, the statement that diseases of the aged are characterized by chronicity, duplicity, and multiplicity is most appro priate in regard to the gastrointestinal tract. Functional bowel distress represents the most common gastrointestinal disorder in the elderly. Indeed, over one-half of all their gastrointestinal complaints are of a functional nature. In view of the many stressful situations confronting elderly patients, such as loss of loved ones, the fears of helplessness, insolvency, ill health, and retirement, it is a marvel that more do not have functional complaints, become depressed, or overcompensate with alcohol. These, of course, make the diagnosis of organic complaints all the more difficult in the geriatric patient. In this chapter, we shall deal primarily with organic diseases afflicting the gastrointestinal tract of the elderly. To do otherwise would require the creation of a sizable textbook. THOMAS W. SHEEHY • Birmingham Veterans Administration Medical Center; and University of Alabama in Birmingham, School of Medicine, Birmingham, Alabama 35233. 63 S. R. Gambert (ed.), Contemporary Geriatric Medicine © Plenum Publishing Corporation 1988 64 THOMAS W. SHEEHY 2.1.1. Pathophysiologic Changes Age leads to general and specific changes in all the organs of the gastrointestinal tract'! Invariably, the teeth show evidence of wear, dis cloration, plaque, and caries. After age 70 years the majority of the elderly are edentulous, and this may lead to nutritional problems. -

Esophogeal Strictures
ESOPHOGEAL STRICTURES Esophageal stricture (ES) is a narrowing in the esophagus – the muscular tube that carries food and liquids from the mouth to the stomach. Most common in recessive dystrophic and junctional EB. Narrowed esophagus makes it difficult to swallow food and sometimes liquid. Major cause of poor nutrition in recessive dystrophic and junctional EB. Not only affects the intake of nutrients but also limits food choice – often times the patients favorite foods are removed from the diet affecting enjoyment of eating and quality of life. How does an ES Esophagus in individuals with dystrophic and junctional EB has extremely fragile form? surface lining and makes it easy for it to blister in response to even the most minor trauma. Blistering can lead to the formation of scar tissue in the wall of the esophagus and can cause it to narrow or even get blocked. Can begin in childhood and risk increases as the patient gets older. Symptoms Difficulty swallowing (dysphagia) Pain with swallowing Weight loss or difficulty gaining weight and poor growth Regurgitation of food, when food comes back into the mouth from above the stricture Food gets stuck in the esophagus (food impaction) Frequent burping or hiccups Heartburn (burning sensation behind the breast plate bone) Tests Barium swallow test: For this test the patient swallows liquid barium, which coats and fills the esophagus, so that it shows up on X-ray images. X-ray pictures are then taken and the radiologist can see if there is a narrowing in the esophagus. Barium is nontoxic and is often flavored to improve the taste. -

Gastroenterology - Outpatient
Gastroenterology - Outpatient Goal Gastroenterology encompasses the evaluation and treatment of patients with disorders of the gastrointestinal tract, pancreas, biliary tract, and liver. It includes disorders of organs within the abdominal cavity and requires knowledge of the manifestations of gastrointestinal disorders in other organ systems, such as the skin. Additional areas include knowledge of nutrition and nutritional deficiencies, and screening and prevention, particularly for colorectal cancer. The general internist should have a wide range of competency in gastroenterology and should be able to provide primary and in some cases secondary preventive care, evaluate a broad array of gastrointestinal symptoms, and manage many gastrointestinal disorders. The general internist is not expected to perform most technical procedures with the important exception of flexible sigmoidoscopy. However, he or she must be familiar with the indications, contraindications, interpretation, and complications of these procedures. Lead Faculty Grace Elta, MD Objectives 1 0 Patient Care and Medical Knowledge 1 1 Dysphagia Differentiate oropharyngeal from esophageal Know the general approach to diagnosis Oropharyngeal dysphagia Use of barium esophagogram/swallowing study Use of endoscopy Use of ENT/speech pathology Know the general approach esophageal dysphagia Use of endoscopy Use of barium esophagogram Know causes of esophageal dysphagia Rings GERD Stricture Pill esophagitis Cancer Know when to include radiology, gastroenterology 1 2 Gastroesophageal -

Options for Treating Pain in Cancer Patients with Dysphagia
Drugs DOI 10.1007/s40265-017-0710-8 THERAPY IN PRACTICE Options for Treating Pain in Cancer Patients with Dysphagia Sebastiano Mercadante1 Ó Springer International Publishing Switzerland 2017 Abstract Patients with chronic pain often develop dys- phagia during the course of an advanced disease such as Key Points cancer. Opioids are the cornerstone of the management of cancer pain and are commonly administered orally. How- The oral route is often not available for opioid ever, the oral route does not suit patients with dysphagia, administration in cancer patients due to dysphagia who require alternative methods to administer analgesic and thus alternative methods should be offered. drugs. Opioids given by parenteral or transdermal routes provide adequate pain control, being at least as efficacious Opioids administered via transdermal and parenteral as the oral route, but knowledge and experience in con- routes may provide efficient analgesia. version ratios are mandatory when using these routes of New technologies may be effective for administration. For breakthrough pain, transmucosal fen- administration of drugs, even in patients who have tanyl preparations should be the preferred option and these difficulties swallowing. can be given as needed due to the route of absorption. In addition, a new class of opioid formulations has been developed for use in dysphagic patients that are adminis- tered via nasogastric or enteral tubes while maintaining their sustained-release properties. 1 Introduction Dysphagia is a swallowing disturbance associated with many neuromuscular conditions and the consequences of systemic weakness. It is a difficulty in swallowing and trouble passing food or liquid down the throat. Some people may gag, cough, or choke when trying to swallow, while others may feel like food is stuck in their throat. -

Dysphagia What Is Dysphagia? Dysphagia Is a General Term Used to Describe Difficulty Swallowing
Dysphagia What is Dysphagia? Dysphagia is a general term used to describe difficulty swallowing. While swallowing may seem very involuntary and basic, it’s actually a rather complex process involving many different muscles and nerves. Swallowing happens in 3 different phases: Insert Shutterstock ID: 119134822 1. During the first phase or oral phase the tongue moves food around in your mouth. Chewing breaks food down into smaller pieces, and saliva moistens food particles and starts to chemically break down our food. 2. During the pharyngeal phase your tongue pushes solids and liquids to the back of your mouth. This triggers a swallowing reflex that passes food through your throat (or pharynx). Your pharynx is the part of your throat behind your mouth and nasal cavity, it’s above your esophagus and larynx (or voice box). During this reflex, your larynx closes off so that food doesn’t get into your airways and lungs. 3. During the esophageal phase solids and liquids enter the esophagus, the muscular tube that carries food to your stomach via a series of wave-like muscular contractions called peristalsis. Insert Shutterstock ID: 1151090882 When the muscles and nerves that control swallowing don’t function properly or something is blocking your throat or esophagus, difficulty swallowing can occur. There are varying degrees of Dysphagia and not everyone will describe the same symptoms. Your symptoms will depend on your specific condition. Some people will experience difficulty swallowing only solids, or only dry solids like breads, while others will have problems swallowing both solids and liquids. Still others won’t be able to swallow anything at all. -

Dysphagia: Evaluation and Collaborative Management
Dysphagia: Evaluation and Collaborative Management John M. Wilkinson, MD; Don Chamil Codipilly, MD; and Robert P. Wilfahrt, MD Mayo Clinic College of Medicine and Science, Rochester, Minnesota Dysphagia is common but may be underreported. Specific symptoms, rather than their perceived location, should guide the initial evaluation and imaging. Obstructive symptoms that seem to originate in the throat or neck may actually be caused by distal esophageal lesions. Oropharyngeal dysphagia manifests as difficulty initiating swallowing, coughing, choking, or aspiration, and it is most commonly caused by chronic neurologic conditions such as stroke, Parkinson disease, or demen- tia. Symptoms should be thoroughly evaluated because of the risk of aspiration. Patients with esophageal dysphagia may report a sensation of food getting stuck after swallowing. This condition is most commonly caused by gastroesophageal reflux disease and functional esophageal disorders. Eosinophilic esophagitis is triggered by food allergens and is increasingly prevalent; esophageal biopsies should be performed to make the diagnosis. Esophageal motility disorders such as achalasia are relatively rare and may be overdiagnosed. Opioid-induced esophageal dysfunction is becoming more common. Esoph- agogastroduodenoscopy is recommended for the initial evaluation of esophageal dysphagia, with barium esophagography as an adjunct. Esophageal cancer and other serious conditions have a low prevalence, and testing in low-risk patients may be deferred while a four-week trial of acid-suppressing therapy is undertaken. Many frail older adults with progressive neuro- logic disease have significant but unrecognized dysphagia, which significantly increases their risk of aspiration pneumonia and malnourishment. In these patients, the diagnosis of dysphagia should prompt a discussion about goals of care before potentially harmful interventions are considered. -

00 PWG-Titledisc.Fm
ICD-10-CM Coding Workbook for General Surgery Specialty coding guidance for ICD-10-CM 2016 Contents Introduction .............................................................................................................................................. 1 Overview of ICD-10 ..............................................................................................................................................................................................1 Getting Ready for ICD-10 ................................................................................................................................................................................... 2 Using This ICD-10-CM Workbook .....................................................................................................................................................................2 Workbook Guidelines ..........................................................................................................................................................................................3 Summary ................................................................................................................................................................................................................4 Case Studies and Questions ...................................................................................................................... 5 Case Study #1—Laparoscopic Appendectomy ............................................................................................................................................5 -

Esophageal Disease.Pdf
● Objectives: ● Know the definition of dysphagia. ● Recognize the causes and types of dysphagia. ● Diagnose the important esophageal diseases like GERD, Achalasia & its major clinical presentations and complications. ● Understand the pathway of investigating patients with dysphagia. ● List the management outline for achalasia, GERD and Ca esophagus . [ Color index : Important | Notes | Extra ] [ Editing file | Feedback | Share your notes | Shared notes | Twitter ] ● Resources: ● 435 slides ● Done by: Mana Almuhaideb & Zaki Alwatban ● Team sub-leader: Jwaher Alharbi ● Team leaders: Khawla AlAmmari & Fahad AlAbdullatif ● Revised by: Ahmad Alyahya & Luluh Alzeghayer Know the definition of dysphagia - First, Swallowing occurs through three phases: 1/Oral phase 2/Pharyngeal phase 3/Esophageal phase - The esophagus is a fibromuscular tube (upper third is composed of skeletal muscles, and the rest is composed of smooth muscles) - It has two sphincters ( UES: upper esophageal sphincter , LES: lower esophageal sphincter) - Esophagus has two main functions: 1- Transport of food by peristalsis 2- Prevention of gastric regurgitation by LES/UES - It is supplied by the vagus nerve & sympathetic trunk - Dysphagia is difficulty in swallowing and suggests an abnormality in the passage of liquids or solids from the oral cavity through the esophagus and into the stomach, has mechanical and neuromuscular causes. - Odynophagia is painful swallowing. - Both dysphagia and odynophagia will result in weight loss ,eventually. Recognize the causes and types of dysphagia. Dysphagia can be either oropharyngeal or esophageal: Oropharyngeal dysphagia Esophageal dysphagia - Also called: transfer dysphagia - Arises from abnormalities in: - Arises from abnormalities of 1-The esophageal body muscles, nerves or structures of the 2-Lower esophageal sphincter 1-Oral cavity 3-Cardia (part of the stomach where the esophagus enters) 2-Pharynx 3-Upper esophagus Classified into mechanical and motor: 4-Upper esophageal sphincter.