Short Communication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entomology 101 Jason J
Entomology 101 Jason J. Dombroskie Manager, Cornell U. Insect Collection Coordinator, Insect Diagnostic Lab This material [email protected] can only be used for CCE MGV audiences. Outline • What is an insect? • Anatomy • Life cycles • Diversity • Major orders • Herbivory Corydalus cornutus Cornell U. Insect Collection • > 7 million specimens • ~200 000 species • worldwide coverage • http://cuic.entomology.cornell.edu/ • on facebook Insect Diagnostic Lab • ~700 IDs per year • 10-20 000 IDs for NYS Dept. Ag. & Markets • occasionally IDs can be made from a photo • mostly local, but some submissions worldwide • $25 fee • http://entomology.cornell.edu/IDL Arthropods Regier, et al. 2005 What is an insect? • 3 main body parts • 6 jointed legs • 1 pair of antennae • compound eyes • usually some sort of metamorphosis Booneacris glacialis Head • antennae • mouthparts • compound eyes • ocelli Monochamus scutellatus Popillia japonica Tetanocera sp. Antheraea polyphemus wikimedia commons labrum maxilla mandible labium Corydalus cornutus Polygonia progne Aedes sp. Hybomitra zonalis Monochamus notatus Aeshna canadensis Isoptera Darapsa myron Thorax • six legs • four wings or less • muscular Amateur Entomologists’ Society Entomologists’ Amateur Limenitis archippus Lethocerus americanus Zeugomantispa minuta Machimus sp. with Herpetogramma pertextalis wikimedia commons Tipula apicalis Cybister fimbriolatus Elasmucha lateralis Automeris io Abdomen • internal organs • genitalia • ovipositor Ophiogomphus rupinsulensis Lauxania shewelli Merope tuber Adoxophyes -

Aquatic Insects Are Dramatically Underrepresented in Genomic Research
insects Communication Aquatic Insects Are Dramatically Underrepresented in Genomic Research Scott Hotaling 1,* , Joanna L. Kelley 1 and Paul B. Frandsen 2,3,* 1 School of Biological Sciences, Washington State University, Pullman, WA 99164, USA; [email protected] 2 Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT 84062, USA 3 Data Science Lab, Smithsonian Institution, Washington, DC 20002, USA * Correspondence: [email protected] (S.H.); [email protected] (P.B.F.); Tel.: +1-(828)-507-9950 (S.H.); +1-(801)-422-2283 (P.B.F.) Received: 20 August 2020; Accepted: 3 September 2020; Published: 5 September 2020 Simple Summary: The genome is the basic evolutionary unit underpinning life on Earth. Knowing its sequence, including the many thousands of genes coding for proteins in an organism, empowers scientific discovery for both the focal organism and related species. Aquatic insects represent 10% of all insect diversity, can be found on every continent except Antarctica, and are key components of freshwater ecosystems. However, aquatic insect genome biology lags dramatically behind that of terrestrial insects. If genomic effort was spread evenly, one aquatic insect genome would be sequenced for every ~9 terrestrial insect genomes. Instead, ~24 terrestrial insect genomes have been sequenced for every aquatic insect genome. A lack of aquatic genomes is limiting research progress in the field at both fundamental and applied scales. We argue that the limited availability of aquatic insect genomes is not due to practical limitations—small body sizes or overly complex genomes—but instead reflects a lack of research interest. We call for targeted efforts to expand the availability of aquatic insect genomic resources to empower future research. -

C:\Documents and Settings\Rheitzma\My Documents
State of Ohio Environmental Protection Agency Division of Surface Water Biological and Water Quality Study of the Big Darby Creek Watershed, 2001/2002 Logan, Champaign, Union, Madison, Franklin and Pickaway Counties Greenside Darter Central Mottled Sculpin (Cottus bairdi bairdi) (Etheostoma blennioides Clubshell Mussel blennioides) (Pleurobema clava) Spotted Darter Eastern Banded Darter (Etheostoma maculatum) (Etheostoma zonale zonale) Helgrammite (Corydalus cornutus) Smallmouth Bass (Micropterus doloieui dolomieui) stonefly nymph mayfly nymph (Isonychia sp.) Bob Taft, Governor Christopher Jones, Director Biological and Water Quality Study of Big Darby Creek and Selected Tributaries 2001/2002 Logan, Champaign, Union, Madison, Franklin and Pickaway Counties, Ohio June 2004 Ohio EPA Technical Report EAS/2004-6-3 prepared by State of Ohio Environmental Protection Agency Division of Surface Water Lazarus Government Center 122 South Front St., Columbus OH 43215 Mail to: P.O. Box 1049, Columbus OH 43216-1049 Bob A Taft Governor, State of Ohio Christopher Jones Director, Ohio Environmental Protection Agency CONTENTS How To Use This Document.................................................. vii Notice to Users.............................................................. viii Acknowledgments .............................................................x Foreword ................................................................... xi Section A. General Study Discussion and Results A.1 Introduction......................................................... -

Hundreds of Species of Aquatic Macroinvertebrates Live in Illinois In
Illinois A B aquatic sowbug Asellus sp. Photograph © Paul P.Tinerella AAqquuaattiicc mayfly A. adult Hexagenia sp.; B. nymph Isonychia sp. MMaaccrrooiinnvveerrtteebbrraatteess Photographs © Michael R. Jeffords northern clearwater crayfish Orconectes propinquus Photograph © Michael R. Jeffords ruby spot damselfly Hetaerina americana Photograph © Michael R. Jeffords aquatic snail Pleurocera acutum Photograph © Jochen Gerber,The Field Museum of Natural History predaceous diving beetle Dytiscus circumcinctus Photograph © Paul P.Tinerella monkeyface mussel Quadrula metanevra common skimmer dragonfly - nymph Libellula sp. Photograph © Kevin S. Cummings Photograph © Paul P.Tinerella water scavenger beetle Hydrochara sp. Photograph © Steve J.Taylor devil crayfish Cambarus diogenes A B Photograph © ChristopherTaylor dobsonfly Corydalus sp. A. larva; B. adult Photographs © Michael R. Jeffords common darner dragonfly - nymph Aeshna sp. Photograph © Paul P.Tinerella giant water bug Belostoma lutarium Photograph © Paul P.Tinerella aquatic worm Slavina appendiculata Photograph © Mark J. Wetzel water boatman Trichocorixa calva Photograph © Paul P.Tinerella aquatic mite Order Prostigmata Photograph © Michael R. Jeffords backswimmer Notonecta irrorata Photograph © Paul P.Tinerella leech - adult and young Class Hirudinea pygmy backswimmer Neoplea striola mosquito - larva Toxorhynchites sp. fishing spider Dolomedes sp. Photograph © William N. Roston Photograph © Paul P.Tinerella Photograph © Michael R. Jeffords Photograph © Paul P.Tinerella Species List Species are not shown in proportion to actual size. undreds of species of aquatic macroinvertebrates live in Illinois in a Kingdom Animalia Hvariety of habitats. Some of the habitats have flowing water while Phylum Annelida Class Clitellata Family Naididae aquatic worm Slavina appendiculata This poster was made possible by: others contain still water. In order to survive in water, these organisms Class Hirudinea leech must be able to breathe, find food, protect themselves, move and reproduce. -
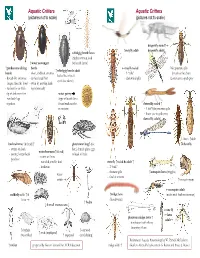
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Ag. Ento. 3.1 Fundamentals of Entomology Credit Ours: (2+1=3) THEORY Part – I 1
Ag. Ento. 3.1 Fundamentals of Entomology Ag. Ento. 3.1 Fundamentals of Entomology Credit ours: (2+1=3) THEORY Part – I 1. History of Entomology in India. 2. Factors for insect‘s abundance. Major points related to dominance of Insecta in Animal kingdom. 3. Classification of phylum Arthropoda up to classes. Relationship of class Insecta with other classes of Arthropoda. Harmful and useful insects. Part – II 4. Morphology: Structure and functions of insect cuticle, moulting and body segmentation. 5. Structure of Head, thorax and abdomen. 6. Structure and modifications of insect antennae 7. Structure and modifications of insect mouth parts 8. Structure and modifications of insect legs, wing venation, modifications and wing coupling apparatus. 9. Metamorphosis and diapause in insects. Types of larvae and pupae. Part – III 10. Structure of male and female genital organs 11. Structure and functions of digestive system 12. Excretory system 13. Circulatory system 14. Respiratory system 15. Nervous system, secretary (Endocrine) and Major sensory organs 16. Reproductive systems in insects. Types of reproduction in insects. MID TERM EXAMINATION Part – IV 17. Systematics: Taxonomy –importance, history and development and binomial nomenclature. 18. Definitions of Biotype, Sub-species, Species, Genus, Family and Order. Classification of class Insecta up to Orders. Major characteristics of orders. Basic groups of present day insects with special emphasis to orders and families of Agricultural importance like 19. Orthoptera: Acrididae, Tettigonidae, Gryllidae, Gryllotalpidae; 20. Dictyoptera: Mantidae, Blattidae; Odonata; Neuroptera: Chrysopidae; 21. Isoptera: Termitidae; Thysanoptera: Thripidae; 22. Hemiptera: Pentatomidae, Coreidae, Cimicidae, Pyrrhocoridae, Lygaeidae, Cicadellidae, Delphacidae, Aphididae, Coccidae, Lophophidae, Aleurodidae, Pseudococcidae; 23. Lepidoptera: Pieridae, Papiloinidae, Noctuidae, Sphingidae, Pyralidae, Gelechiidae, Arctiidae, Saturnidae, Bombycidae; 24. -

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

A New Fishfly Species (Megaloptera: Corydalidae: Chauliodinae) from Eocene Baltic Amber
Palaeoentomology 003 (2): 188–195 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY Copyright © 2020 Magnolia Press Article ISSN 2624-2834 (online edition) PE https://doi.org/10.11646/palaeoentomology.3.2.8 http://zoobank.org/urn:lsid:zoobank.org:pub:20A34D9A-DC69-453E-9662-0A8FAFA25677 A new fishfly species (Megaloptera: Corydalidae: Chauliodinae) from Eocene Baltic amber XINGYUE LIU1, * & JÖRG ANSORGE2 1College of Life Science and Technology, Hubei Engineering University, Xiaogan 432000, China �[email protected]; https://orcid.org/0000-0002-9168-0659 2Institute of Geography and Geology, University of Greifswald, Friedrich-Ludwig-Jahnstraße 17a, D-17487 Greifswald, Germany �[email protected]; https://orcid.org/0000-0002-1284-6893 *Corresponding author. �[email protected] Abstract and Sialidae (alderflies). Species of Megaloptera have worldwide distribution, but most of them occur mainly in The fossil record of Megaloptera (Insecta: Holometabola: subtropical and warm temperate regions, e.g., the Oriental, Neuropterida) is very limited. Both megalopteran families, i.e., Corydalidae and Sialidae, have been found in the Eocene Neotropical, and Australian Regions (Yang & Liu, 2010; Baltic amber, comprising two named species in one genus Liu et al., 2012, 2015a). The phylogeny and biogeography of Corydalidae (Chauliodinae) and four named species in of extant Megaloptera have been intensively studied in two genera of Sialidae. Here we report a new species of Liu et al. (2012, 2015a, b, 2016) and Contreras-Ramos Chauliodinae from the Baltic amber, namely Nigronia (2011). prussia sp. nov.. The new species possesses a spotted hind Compared with the other two orders of Neuropterida wing with broad band-like marking, a well-developed stem (Raphidioptera and Neuroptera), the fossil record of of hind wing MA subdistally with a short crossvein to MP, a Megaloptera is considerably scarce. -

Living Water. Eno River State Park: an Environmental Education Learning Experience Designed for the Middle Grades. INSTITUTION North Carolina State Dept
DOCUMENT RESUME ED 376 024 SE 054 365 AUTHOR Hartley, Scott; Woods, Martha TITLE Living Water. Eno River State Park: An Environmental Education Learning Experience Designed for the Middle Grades. INSTITUTION North Carolina State Dept. of Environment, Health, and Natural Resources, Raleigh. Div. of Parks and Recreation. PUB DATE Oct 92 NOTE 96p.; For other Environmental Education Learning Experiences, see SE 054 364-371. AVAILABLE ,FROM North Carolina Division of Parks and Recreation, P.O. Box 27687, Raleigh, NC 27611-7687. PUB TYPE Guides Classroom Use Teaching Guides (For Teacher)(052) EDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS Classification; Computation; Ecology; Entomology; Environmental Education; Experiential Learning; Field Trips; Grade 5; Grade 6; Integrated Activities; Intermediate Grades; Maps; *Marine Biology; Natural Resources; *Outdoor Activities; *Outdoor Education; Teaching Guides; Water Pollution; *Water Quality; *Water Resources IDENTIFIERS Dichotomous Keys; Environmental. Management; *North Carolina; pH; Rivers; State Parks; Water Quality Analysis; Watersheds ABSTRACT This learning packet, one in a series of eight, was developed by the Eno River State Park in North Carolina for Grades 5-6 to teach about various aspects of water life on the Eno River. Loose -leaf pages are presented in nine sections that contain: (1) introductions to the North Carolina State Park System, the Eno River State Park, and to the park's activity packet;(2) a summary of the activities that includes major concepts and objectives covered; (3) pre-visit activities on map trivia and dichotomous classification keys;(4) on-site activities on river flow, pH values, water bugs and river sediment;(5) post-visit activities on water pollution; (6)a list ol7 69 related vocabulary words; (7) park and parental permission forms for the visit; and (8) blank pages for taking notes. -

The Musculature of the Head of the Corydalus Larva (Neuroptera, Sialidae)
THE MUSCULATURE 0%’ THE HEAD OF THE CORYDALUS LARVA (NEURqOPTERA, SIALIDAE) 1,2 SOL KBAMER * Departmawt of Zoology, University of Wisconsm, Madison, Wisconsin ‘IWENTY-EIGIXT FIGURES The Neuroptera have long been regarded one of the most primitive groups of the holometabolous insects. Crampton ( ’21) considered the Neuroptera an extremely important group from the standpoint of the siudy of the phylogeny of higher in- sects, and emphasized the primitiveness of such forms as Sialis, Corydalus, and Chauliodea among the Neuroptera (Megaloptera). Although he considered the Coleoptera the more primitive of the two orders from a consideration of the head and mouthparts, he emphasized the astonishing similarity between larval Coleoptera and Neuroptera and concluded that the Coleoptera appear to lead to the Neuroptera which in turn lead to the Mecoptera, Diptera and Siphonaptera on the one hand and to the Trichoptera and Lepidoptera on the other. More recently Chen (’46),in a study on the evolution of insect larvae based on a wide series throughout the Holometabola, selected the nenropteroid genus Corydalus as possessing the most primitive or generalized known type of larva. He desig- nated this primitive type by the term “campodeoid-polypod, ’? characterized by having three pairs of thoracic and 10 pairs of abdominal legs, the latter bearing each a vesicle and a stylus. From this study Chen concluded that all existing types of larvae have been (derivedfrom this primitive type in two ways : Corydalus cornutus L. The writer is grateful for the assistance of R. E. Snodgrass in whose laboratory at the U. S. National Museum, Washington, D. C. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Primeiro Registro De Corydalus Peruvianus Davis (Megaloptera: Coridalidae: Coridalinae) No Brasil
doi:10.12741/ebrasilis.v11i1.704 e-ISSN 1983-0572 Publication of the project Entomologistas do Brasil www.ebras.bio.br Creative Commons Licence v4.0 (BY-NC-SA) Copyright © EntomoBrasilis Copyright © Author(s) Scientific Note/Comunicação Científica Primeiro registro de Corydalus peruvianus Davis (Megaloptera: Coridalidae: Coridalinae) no Brasil Registered on ZooBank: urn:lsid:zoobank.org:pub:ADC79A4E-33D7-494E-B2EC-39AFA4E17042 Jaime de Liege Gama Neto¹ & Mahedy Araujo Bastos Passos² 1. Universidade Estadual de Roraima (UERR). 2. Centro Estadual de Educação Profissional Prof. Antônio de Pinho Lima. EntomoBrasilis 11 (1): 45-48 (2018) Resumo. Registra-se pela primeira vez no Brasil o megaloptera Corydalus peruvianus Davis, ampliando a distribuição geográfica da espécie na América do Sul. Adicionalmente, são fornecidas informações sobre o ambiente e a qualidade da água do local onde a espécie foi coletada. Palavras-Chave: América do Sul; Distribuição geográfica; Insetos aquáticos; Roraima; Serra do Tepequém. First record of Corydalus peruvianus Davis (Megaloptera: Coridalidae: Coridalinae) from Brazil Abstract. We recorded, for the first time in Brazil, the megalopteran Corydalus peruvianus Davis, expanding their geographic distribution in the South America. We complement this information with data on the environment and water quality of the locality where the species was collected. Keywords: Aquatic insects; Geographic distribution; South America; Tepequém mountain range; Roraima. egaloptera é uma pequena ordem de insetos aquáticos Dentro desse contexto, continuando o esforço para descrever holometábolos, com aproximadamente 348 espécies e registrar a fauna de insetos aquáticos do estado de Roraima, mundialmente distribuídas (CONTRERAS -RAMOS 2011), o presente trabalho tem como objetivo apresentar o primeiro caracterizados por um estágio larval exclusivamente aquático registro de Corydalus peruvianus Davis no Brasil, ampliando a e por adultos alados com cabeça prognata e área anal da asa distribuição geográfica da espécie na América do Sul.