ABSTRACT GUPTA, AMIT. Improving UV Resistance of High Strength Fibers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications
Graduate Theses, Dissertations, and Problem Reports 2010 Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications Timothy L. Weadon Jr. West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Weadon, Timothy L. Jr., "Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications" (2010). Graduate Theses, Dissertations, and Problem Reports. 4669. https://researchrepository.wvu.edu/etd/4669 This Thesis is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Long Term Loading and Additional Material Properties of Vectran Fabric for Inflatable Structure Applications Timothy L Weadon, Jr Thesis submitted to the College of Engineering and Mineral Resources at West Virginia University in partial fulfillment of the requirements -

A Perspective on High-Performance CNT Fibres for Structural Composites
A Perspective on High-performance CNT fibres for Structural Composites Anastasiia Mikhalchan, Juan José Vilatela IMDEA Materials Institute, C/Eric Kandel 2, Getafe, Madrid, Spain, 28906 This review summarizes progress on structural composites with carbon nanotube (CNT) fibres. It starts by analyzing their development towards a macroscopic ensemble of elongated and aligned crystalline domains alongside the evolution of the structure of traditional high- performance fibres. Literature on tensile properties suggests that there are two emerging grades: highly aligned fibres spun from liquid crystalline solutions, with high modulus (160 GPa/SG) and strength (1.6 GPa/SG), and spun from aerogels of ultra-long nanotubes, combining high strength and fracture energy (up to 100J/g). The fabrication of large unidirectional fabrics with similar properties as the fibres is presently a challenge, which CNT alignment remaining a key factor. A promising approach is to produce fabrics directly from aerogel filaments without having to densify and handle individual CNT fibres. Structural composites of CNT fibres have reached longitudinal properties of about 1 GPa strength and 140 GPa modulus, however, on relatively small samples. In general, there is need to demonstrate fabrication of large CNT fibre laminate composites using standard fabrication routes and to study longitudinal and transverse mechanical properties in tension and compression. Complementary areas of development are interlaminar reinforcement Corresponding Author. E-mail: [email protected] (Anastasiia Mikhalchan) Corresponding Author. E-mail: [email protected] (Juan José Vilatela) 1 with CNT fabric interleaves, and multifunctional structural composites with energy storage or harvesting functions. 2 TABLE OF CONTENTS PART 1. State-of-the-art of CNT fibres 5 1.1 Synthesis and hierarchical structure of CNT fibres 5 1.2 Mechanical properties of CNT fibres 8 1.3 Historical retrospective on R&D of commercial high-performance fibres and CNT 11 fibres PART 2. -

Industrial Fabric Yarn Fibers Processes Products
MOVING HIGH PERFORMANCE FIBERS FORWARD INDUSTRIAL FABRIC YARN FIBERS PROCESSES PRODUCTS WHY FIBER-LINE®FIBER INDUSTRIAL OPTICAL FABRIC CABLES YARN? Key Features Overview • Available Flat or Twisted • FIBER-LINE®’s offering of Industrial Fabric Yarn are utilized in a wide • Material can be “Rolled flat” to remove range of applications. FIBER-LINE®’s marriage of high-performance false or producers’ twist fibers and additional processing create unique products that extend • Metered lengths and various spool sizes the life of our customer’s end use product. available • Our yarn have been processed in a variety of different ways utilizing: Knitting, weaving, braiding. FIBER-LINE® FIBERS FOR INDUSTRIAL FABRIC YARN Packaging • Kevlar® Para-Aramid FIBER-LINE® Industrial Fabric Yarn are supplied on a variety of card- • Nomex® Meta-Aramid board tubes to meet your equipment needs. Contact us today • Vectran® Liquid Crystal Polymer (LCP) with tube dimensions you require. Packages can be supplied on colored, • Carbon Fiber embossed and/or slit tubes. Plastic or metal reels are also available. • Fiberglass • Technora® FIBER-LINE® PERFORMANCE ADDING COATINGS • FIBER-LINE® Colorcoat™: Vibrant Colors for Aesthetic Applications • FIBER-LINE® Wearcoat™: Abrasion & High-Temperature Resistance • FIBER-LINE® Bondcoat™: Adhesion Promotion • FIBER-LINE® Repelcoat™: Water Repellancy • FIBER-LINE® Protexcoat™: UV Degradation Prevention MOVING HIGH PERFORMANCE FIBERS FORWARD POPULAR INDUSTRIAL FABRIC YARN PRODUCTS FIBER-LINE® Nominal Product ID Base Fiber Nominal -

Temperature and Humidity Aging of Poly(P-Phenylene-2,6
Polymer Degradation and Stability 92 (2007) 1234e1246 www.elsevier.com/locate/polydegstab Temperature and humidity aging of poly( p-phenylene-2,6- benzobisoxazole) fibers: Chemical and physical characterization Joannie Chin a,*, Amanda Forster b, Cyril Clerici a, Lipiin Sung a, Mounira Oudina a, Kirk Rice b a Polymeric Materials Group, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States b Office of Law Enforcement Standards, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States Received 9 February 2007; received in revised form 20 March 2007; accepted 23 March 2007 Available online 19 April 2007 Abstract In recent years, poly( p-phenylene-2,6-benzobisoxazole) (PBO) fibers have become prominent in high strength applications such as body ar- mor, ropes and cables, and recreational equipment. The objectives of this study were to expose woven PBO body armor panels to elevated tem- perature and moisture, and to analyze the chemical, morphological and mechanical changes in PBO yarns extracted from the panels. A 30% decrease in yarn tensile strength, which was correlated to changes in the infrared peak absorbance of key functional groups in the PBO structure, was observed during the 26 week elevated temperature/elevated moisture aging period. Substantial changes in chemical structure were observed via infrared spectroscopy, as well as changes in polymer morphology using microscopy and neutron scattering. When the panels were removed to an ultra-dry environment for storage for 47 weeks, no further decreases in tensile strength degradation were observed. In a follow-on study, fibers were sealed in argon-filled glass tubes and exposed to elevated temperature; less than a 4% decrease in tensile strength was observed after 30 weeks, demonstrating that moisture is a key factor in the degradation of these fibers. -

High Performance Fibers
High Performance Fibers One step ahead High Performance Fibers are engineered for extreme uses; whether the requirement is exceptional strength, stiffness, heat resistance and/or chemical resistance. EuroFibers is proud distribution partner of the leading brands in this industry with the ability to tailor these tough fibers to the need of our customers, whether it be coating, twisting, assembling, plying or cutting. HMPE Fiber Para-Aramid Fiber Ultra high molecular weight polyethylene (UHMwPE), high modulus Para-aramid fibers are a class of heat-resistant and extremely strong polyethylene (HMPE) or high performance polyethylene fibers (HPPE) synthetic fibers. The ultimate strength of some aramid fibers can are extremely strong and are the lightest of all ultra-strong fibers. exceed 3500 MPa. Aramid has an outstanding strength-to-weight The ultimate strength can exceed 3000 MPa. However, due to its low ratio, even better than carbon, and excellent dimensional stability melting point of about 150°C (295°F) they are not suitable for elevated due to the high young’s modulus. Para-aramid has a decomposition temperature applications. The fiber is mainly used in protective temperature of ± 500 ºC. Technora® is a para-aramid fiber made clothing like ballistic vests, helmets, cut-resistant glove and tension from copolymers and is produced in the different process from PPTA members like ropes, slings and fishing lines. (poly-paraphenylene terephthalamide). EuroFibers is the premium distributor of DSM offering the extensive EuroFibers is the premium distributor of Teijin® offering their Dyneema® and Trevo® portfolio to our customer base. exceptional aramid fibers Twaron® and Technora® to a wide variety of customers. -
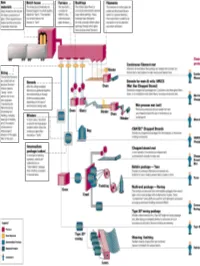
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Cryogenic Temperature Effects on the Mechanical Properties
CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Mechanical Engineering in the Department of Mechanical Engineering Mississippi State, Mississippi May 2008 CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings Approved: ______________________________ ______________________________ Judy A. Schneider Anthony J. Vizzini Associate Professor of Mechanical Professor and Head of Aerospace Engineering Engineering Department (Director of Thesis) (Committee Member) ______________________________ ______________________________ Thomas E. Lacy Steve Daniewicz Associate Professor of Aerospace Professor and Graduate Coordinator Engineering of Mechanical Engineering (Committee Member) Department ______________________________ W.G. Steele Interim Dean and Professor Bagley College of Engineering Name: William Chad Hastings Date of Degree: May 2, 2008 Institution: Mississippi State University Major Field: Mechanical Engineering Major Professor: Judy Schneider Title of Study: CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS Pages in Study: 39 Candidate for Degree of Master of Science This study examines the effects of cryogenic temperatures on the mechanical properties of carbon, aramid, and poly(p-phenylene-2, 6-benzobisoxazole) (PBO) fibers. Although the mechanical properties are documented for these fibers at ambient and elevated temperatures, there is an absence of data in the open literature for how these fibers behave at very low temperatures. To evaluate the mechanical properties, the ASTM standard method for testing at ambient temperature was used as a baseline. The low temperature tests were conducted inside a double walled cryogenic chamber to evaluate the fiber performance at 100K. -

(Pbo) Fibers Under Elevated Temperature and Humidi
FACTORS CONTRIBUTING TO THE DEGRADATION OF POLY(P- PHENYLENE BENZOBISOXAZOLE) (PBO) FIBERS UNDER ELEVATED TEMPERATURE AND HUMIDITY CONDITIONS A Thesis by JOSEPH M. O’NEIL Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2006 Major Subject: Mechanical Engineering FACTORS CONTRIBUTING TO THE DEGRADATION OF POLY(P- PHENYLENE BENZOBISOXAZOLE) (PBO) FIBERS UNDER ELEVATED TEMPERATURE AND HUMIDITY CONDITIONS A Thesis by JOSEPH M. O’NEIL Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Roger Morgan Committee Members, Jaime Grunlan Michael Bevan Head of Department, Dennis O’Neal August 2006 Major Subject: Mechanical Engineering iii ABSTRACT Factors Contributing to the Degradation of Poly(p-phenylene benzobisoxazole) (PBO) Fibers under Elevated Temperature and Humidity Conditions. (August 2006) Joseph M. O’Neil, B.S., Texas A&M University Chair of Advisory Committee: Dr. Roger J. Morgan The moisture absorption behavior of Zylon fibers was characterized in various high temperature and high humidity conditions in a controlled environment. The results of these thermal cycling tests show that PBO fibers not only absorb, but also retain moisture (approximately 0.5-3%) when exposed to elevated temperature and humidity cycles. Also, the impurities of Zylon fibers were characterized through the use of Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and solid state Nuclear Magnetic Resonance (NMR). These tests demonstrated that, in addition to other impurities, PBO fibers may contain up to 0.55 weight percent phosphorus, and that this phosphorus is present in the form of phosphoric acid. -
![VECTRAN TECH.FNL[FINAL]-QXD 10/26/99 10:13 AM Page 2](https://docslib.b-cdn.net/cover/3403/vectran-tech-fnl-final-qxd-10-26-99-10-13-am-page-2-1963403.webp)
VECTRAN TECH.FNL[FINAL]-QXD 10/26/99 10:13 AM Page 2
ENGINEERING DATA VECTRAN TECH.FNL[FINAL]-QXD 10/26/99 10:13 AM Page 2 GGrasprasp ThThee WWorldorld OOff TTomorrowomorrow ENGINEERING DATA ENGINEERING DATA VECTRAN TECH.FNL[FINAL]-QXD 10/26/99 10:13 AM Page 3 TABLE OF CONTENTS Page 1: Introduction Page 2: Vectra Polymer Page 3: Tensile Properties Page 4: Thermal Properties Page 6: Chemical Resistance Page 10: Creep Page 11: Abrasion Resistance Page 14: Compression /Flex Fatigue Page 16: Pin Diameter Page 17: Vibration Damping /Impact Resistance Page 19: Cut Resistance Page 19: Twist Page 21: UV Resistance VECTRAN TECH.FNL[FINAL]-QXD 10/26/99 10:13 AM Page 4 ® L IQUID C RYSTAL P OLYMER F IBER Vectran is a high-performance liquid crystal polymer (LCP) fiber offering a balance of properties unmatched by other performance fibers. Some of these unique properties were discovered through 20 years of research and development of the polymer Vectra®. Celanese committed to developing a thermotropic (melt processable) LCP in the mid 1970’s and commercialized the Vectra family of resins in 1985. The molecules of the liquid crystal polymer are rigid and position themselves into randomly oriented domains.The polymer exhibits anisotropic behavior in the melt state, thus the term “liquid crystal polymer.” Upon extrusion of the molten polymer through small spinnerette holes, the molecular domains align parallel to each other along the fiber axis.The highly oriented fiber structure results in high tensile properties. A schematic diagram of the molecular chain structure is shown in Figure 1. Figure 1: Schematic Of Molecular Chain Structure Of Fiber Conventional Liquid Crystal The attributes of Vectra, such as excellent Polyester Polymer mechanical properties, property retention over a wide range of temperatures, excellent chemical resistance, and low moisture pick-up, carry Melt Spinning Melt And Spinning over to the fiber.The fiber also exhibits no Heat Drawing measurable creep when loaded up to 50% of the threadline breaking load. -

Mer Landing.Qxd
NATIONAL AERONAUTICS AND SPACE ADMINISTRATION Mars Exploration Rover Landings Press Kit January 2004 Media Contacts Donald Savage Policy/Program Management 202/358-1547 Headquarters [email protected] Washington, D.C. Guy Webster Mars Exploration Rover Mission 818/354-5011 Jet Propulsion Laboratory, [email protected] Pasadena, Calif. David Brand Science Payload 607/255-3651 Cornell University, [email protected] Ithaca, N.Y. Contents General Release …………………………………………………………..................................…… 3 Media Services Information ……………………………………….........................................…..... 5 Quick Facts ………………………………………………………................................……………… 6 Mars at a Glance ……………………………………………………….................................………. 7 Historical Mars Missions ………………………………………………….....................................… 8 Mars: The Water Trail ………………………………………………………………….................…… 9 Where We've Been and Where We're Going …………………………………................ 14 Science Investigations .............................................................................................................. 17 Landing Sites ............................................................................................................................. 23 Mission Overview ……………...………………………………………..............................………. 28 Spacecraft ................................................................................................................................. 38 Program/Project Management …………………………………………….................................… -

Ballistic Impact Response of Kevlar 49 and Zylon Under Conditions Representing Jet Engine Fan Containment
Ballistic Impact Response of Kevlar 49 and Zylon Under Conditions Representing Jet Engine Fan Containment J. Michael Pereira and Duane M. Revilock NASA Glenn Research Center, Cleveland, Ohio [email protected] [email protected] Abstract A ballistic impact test program was conducted to provide validation data for the development of numerical models of blade out events in fabric containment systems. The impact response of two different fiber materials - Kevlar 49 (E.I. DuPont Nemours and Company) and Zylon AS (Toyobo Co., Ltd.) was studied by firing metal projectiles into dry woven fabric specimens using a gas gun. The shape, mass, orientation and velocity of the projectile were varied and recorded. In most cases the tests were designed such that the projectile would perforate the specimen, allowing measurement of the energy absorbed by the fabric. The results for both Zylon and Kevlar presented here represent a useful set of data for the purposes of establishing and validating numerical models for predicting the response of fabrics under conditions simulating those of a jet engine blade release situation. In addition some useful empirical observations were made regarding the effects of projectile orientation and the relative performance of the different materials. Introduction In the last thirty years the use of aramid fabrics in jet engine blade containment systems has become common. It is recognized that high strength and high elongation fabrics, combined with innovative structural concepts can provide a light weight, effective fan case system that provides the strength required to safely handle impact loads, blade rub loads and the large dynamic loads caused by rotor imbalance. -

Melt-Spun Fibers for Textile Applications
materials Review Melt-Spun Fibers for Textile Applications Rudolf Hufenus 1,*, Yurong Yan 2, Martin Dauner 3 and Takeshi Kikutani 4 1 Laboratory for Advanced Fibers, Empa, Swiss Federal Laboratories for Materials Science and Technology, Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland 2 Key Lab Guangdong High Property & Functional Polymer Materials, Department of Polymer Materials and Engineering, South China University of Technology, No. 381 Wushan Road, Tianhe, Guangzhou 510640, China; [email protected] 3 German Institutes of Textile and Fiber Research, Körschtalstraße 26, D-73770 Denkendorf, Germany; [email protected] 4 Tokyo Institute of Technology, 4259-J3-142, Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan; [email protected] * Correspondence: [email protected]; Tel.: +41-58-765-7341 Received: 19 August 2020; Accepted: 23 September 2020; Published: 26 September 2020 Abstract: Textiles have a very long history, but they are far from becoming outdated. They gain new importance in technical applications, and man-made fibers are at the center of this ongoing innovation. The development of high-tech textiles relies on enhancements of fiber raw materials and processing techniques. Today, melt spinning of polymers is the most commonly used method for manufacturing commercial fibers, due to the simplicity of the production line, high spinning velocities, low production cost and environmental friendliness. Topics covered in this review are established and novel polymers, additives and processes used in melt spinning. In addition, fundamental questions regarding fiber morphologies, structure-property relationships, as well as flow and draw instabilities are addressed. Multicomponent melt-spinning, where several functionalities can be combined in one fiber, is also discussed.