Butadiene Sulfone As ‘Volatile’, Recyclable Dipolar, Aprotic Solvent for Conducting Substitution and Cycloaddition Reactions Yong Huang1,3†, Esteban E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Flyer
NexantThinkingTM Special Reports C5 Chemicals: Unlocking Hidden Opportunities in Steam Crackers? Brochure January 2017 NexantThinkingTM Special Reports C5 Chemicals: Unlocking Hidden Opportunities in Steam Crackers? Brochure January 2017 A50801. Special Reports This Report was prepared by Nexant, Inc. (“Nexant”) and is part of the NexantThinking™ suite. Except where specifically stated otherwise in this Report, the information contained herein is prepared on the basis of information that is publicly available, and contains no confidential third party technical information to the best knowledge of Nexant. Aforesaid information has not been independently verified or otherwise examined to determine its accuracy, completeness or financial feasibility. Neither Nexant, Subscriber nor any person acting on behalf of either assumes any liabilities with respect to the use of or for damages resulting from the use of any information contained in this Report. Nexant does not represent or warrant that any assumed conditions will come to pass. The Report is submitted on the understanding that the Subscriber will maintain the contents confidential except for the Subscriber’s internal use. The Report should not be reproduced, distributed or used without first obtaining prior written consent by Nexant. Each Subscriber agrees to use reasonable effort to protect the confidential nature of the Report. Copyright © by Nexant Inc. 2017. All rights reserved. Contents Section Page 1 Introduction and Report Objectives ....................................................................................... -

SAFETY DATA SHEET Classified in Accordance with Health Canada Hazardous Products Regulations (SOR/2015-17)
Version: 8.0 Revision Date: 01/02/2020 SAFETY DATA SHEET Classified in accordance with Health Canada Hazardous Products Regulations (SOR/2015-17) 1. Identification Product identifier: Hydrocarbons C5 Rich Other means of identification Common name(s), Crude Isoprene; Isoprene/Piperylene Concentrate; C5 Mixture; Isoprene synonym(s): Product SDS number: NOVA-0007 Recommended use and restriction on use Recommended use: Petrochemical feedstock. Restrictions on use: All uses other than the identified. Manufacturer/Importer/Supplier/Distributor Information Manufacturer Company Name: NOVA Chemicals Address: P.O. Box 2518, Station M Calgary, Alberta, Canada T2P 5C6 Telephone: Product Information: 1-412-490-4063 SDS Information Email: [email protected] Emergency telephone number: 1-800-561-6682, 1-403-314-8767 (NOVA Chemicals) (24 hours) 2. Hazard(s) identification Hazard Classification According to Hazardous Products Regulations Physical Hazards Flammable liquids Category 1 Static-accumulating flammable liquid Category 1 Health Hazards Acute toxicity (Oral) Category 4 Skin Corrosion/Irritation Category 2 Serious Eye Damage/Eye Irritation Category 2A Germ Cell Mutagenicity Category 2 Carcinogenicity Category 1B Specific Target Organ Toxicity - Category 3 Single Exposure Aspiration Hazard Category 1 Environmental Hazards Acute hazards to the aquatic Category 3 environment Chronic hazards to the aquatic Category 3 environment Label Elements Hazard Symbol: SDS_CA 1/14 Version: 8.0 Revision Date: 01/02/2020 Signal Word: Danger Hazard Statement: Extremely flammable liquid and vapour. Static accumulating flammable liquid can become electrostatically charged even in bonded and grounded equipment. Sparks may ignite liquid and vapour. May cause flash fire or explosion. Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. -

Material Safety Data Sheet SECTION 1: PRODUCT and COMPANY IDENTIFICATION SECTION 2: INGREDIENTS
MATERIAL SAFETY DATA SHEET 3M™ Weatherstrip Adhesive - Black, P.N. 08011 08/26/11 Material Safety Data Sheet Copyright, 2011, 3M Company All rights reserved. Copying and/or downloading of this information for the purpose of properly utilizing 3M products is allowed provided that: (1) the information is copied in full with no changes unless prior written agreement is obtained from 3M, and (2) neither the copy nor the original is resold or otherwise distributed with the intention of earning a profit thereon. SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: 3M™ Weatherstrip Adhesive - Black, P.N. 08011 MANUFACTURER: 3M DIVISION: Automotive Aftermarket ADDRESS: 3M Center, St. Paul, MN 55144-1000 EMERGENCY PHONE: 1-800-364-3577 or (651) 737-6501 (24 hours) Issue Date: 08/26/11 Supercedes Date: 02/03/09 Document Group: 10-2974-3 Product Use: Intended Use: Automotive Specific Use: Trim Adhesive SECTION 2: INGREDIENTS Ingredient C.A.S. No. % by Wt NAPHTHA (PETROLEUM), SOLVENT-REFINED LIGHT 64741-84-0 40 - 60 HEXANE 110-54-3 10 - 30 HEPTANE 142-82-5 2 - 12 METHYLCYCLOPENTANE 96-37-7 2 - 12 2-METHYLPENTANE 107-83-5 5 - 10 3-METHYLPENTANE 96-14-0 5 - 10 POLYISOPRENE 9003-31-0 3 - 7 NAPHTHA, LIGHT STEAM-CRACKED AROM., PIPERYLENE CONC., 68478-07-9 3 - 7 POLYMD. STYRENE-BUTADIENE POLYMER 9003-55-8 3 - 7 TALC 14807-96-6 3 - 7 TOLUENE 108-88-3 3 - 7 FORMALDEHYDE, POLYMER WITH 4-(1,1-DIMETHYLETHYL)PHENOL, Trade Secret 1 - 5 MAGNESIUM OXIDE COMPLEX ROSIN, POLYMD., CALCIUM AND ZINC SALTS 68188-23-8 0 - 5 ZINC RESINATE 9010-69-9 0 - 3 CYCLOHEXANE 110-82-7 < 3 2,3-DIMETHYLBUTANE 79-29-8 0.5 - 1.5 ETHYL ALCOHOL 64-17-5 0.1 - 1 ZINC OXIDE 1314-13-2 <= 0.23 BENZENE 71-43-2 < 0.06 _________________________________________________________________________________________________ Page 1 of 10 MATERIAL SAFETY DATA SHEET 3M™ Weatherstrip Adhesive - Black, P.N. -

United States Patent' Office
' Patented Feb. 7, i950 - 2,496,868 UNITED STATES PATENT‘ OFFICE 2,496,868 POLYMERS OF 3-VINYLPYRENE Ralph G. Flowers, Pitts?eld, Mass., assignm- to to General Electric Company, a corporation of New York No Drawing. Application May 13, 1948, ' , Serial No. 26,940 12 Claims. (Cl. 260—87.3) 2 This invention relates to polymers and co such as boron tri?uoride, are used. aperiod of polymers of vinyl compounds and to methods of time elapses between the addition of the catalyst , preparing same. More particularly, the inven and the beginning of the polymerization. This tion-ia concerned with new and useful composi tendency is particularly marked when the poly tions of matter comprising the product of poly merization is carried out at elevated tempera merization of a mass comprising 3-vinylpyrene. tures. A very large amount of solvent often in In my copending application, Serial No. 26,939, hibits or retards the polymerization of the mono ?led concurrently herewith and assigned to the mer or comonomers to the extent that polymer same assignee as the present invention, there are ization is not e?ected in a reasonable time. disclosed and claimed 3-vinylpyrene and methods 10 Another method which has been found effective - of preparing the same. I have now discovered in preparing the homopolymers and copolymers that this monomeric material may be polymer of the instant claimed invention includes ‘the ized or copolymerized with other polymerizable preparation of an intimate mixture of the mono compositions to yield valuable and useful com- ' meric materials by preparing a suspension or positions of matter. -

Fullerene-Acene Chemistry
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2007 Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene Andreas John Athans University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Athans, Andreas John, "Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene" (2007). Doctoral Dissertations. 363. https://scholars.unh.edu/dissertation/363 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. FULLERENE-ACENE CHEMISTRY: PART I: STUDIES ON THE REGIOSELECTIVE REDUCTION OF ACENES AND ACENE QUINONES; PART II: PROGRESS TOWARD THE SYNTHESIS OF LARGE ACENES AND THEIR DIELS- ALDER CHEMISTRY WITH [60]FULLERENE VOLUME 1 CHAPTERS 1-5 BY ANDREAS JOHN ATHANS B.S. University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy m Chemistry May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3 2 6 0 5 8 6 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

United States Patent Office Patented Sept
3,205,214 United States Patent Office Patented Sept. 7, 1965 2 usual catalytic amount of the two catalytic components 3,205,214 can be used within the range of, for example, 0.2 to about PPERYLENE POLYMERZATION Thomas B. Taicott, Wadsworth Township, Ohio, assignor 20 millimoles per 100 grams of monomer. to The Firestone Tire & Rubber Company, Akron, Piperylene can be polymerized by the method of the Ohio, a corporation of Ohio 5 invention either as the sole monomer or in the presence No Drawing. Filed May 15, 1961, Ser. No. 109,814 of one or more additional ethylenically unsaturated com 2 Claims. (C. 260-94.3) pound, with which it may or may not copolymerize, de pending upon the specific monomer and the specific cata This invention relates to the polymerization of piperyl lyst combination. For instance, a mixed hydrocarbon ene, either cis-piperylene or trans-piperylene or a mixture O stream containing both piperylene and isoprene can be of the two, and other monomers may be present. The treated to polymerizing conditions in accordance with the invention relates more particularly to a process of polym-. invention to polymerize the piperylene selectively, leaving erization using a catalyst containing iodine, either in com all or most of the isoprene in the stream unpolymerized. bined or initially uncombined form. A different catalyst ratio or combination within the scope A piperylene polymer produced by the invention con 15 of the invention can be utilized to produce a piperylene tain in the piperylene portion over 98 percent 1,4-addi isoprene copolymer. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -
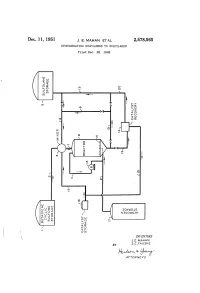
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -

2002-00238-01-E.Pdf (Pdf)
report no. 01/54 environmental classification of petroleum substances - summary data and rationale Prepared by CONCAWE Petroleum Products Ecology Group D. King (Chairman) M. Bradfield P. Falkenback T. Parkerton D. Peterson E. Remy R. Toy M. Wright B. Dmytrasz D. Short Reproduction permitted with due acknowledgement CONCAWE Brussels October 2001 I report no. 01/54 ABSTRACT Environmental data on the fate and effects of petroleum substances are summarised. Technical issues relating to the choice of test methodology for the evaluation of environmental impacts of petroleum substances are discussed. Proposals for self-classification according to EU criteria, as defined in the Dangerous Substances Directive, are presented for individual petroleum substance groups, on the basis of available test data and structure activity relationships (based on composition). KEYWORDS Hazard, environment, petroleum substances, classification, aquatic toxicity, biodegradation, bioaccumulation, QSAR, dangerous substances directive. INTERNET This report is available as an Adobe pdf file on the CONCAWE website (www.concawe.be). NOTE Considerable efforts have been made to assure the accuracy and reliability of the information contained in this publication. However, neither CONCAWE nor any company participating in CONCAWE can accept liability for any loss, damage or injury whatsoever resulting from the use of this information. This report does not necessarily represent the views of any company participating in CONCAWE. II report no. 01/54 CONTENTS Page SUMMARY V 1. REGULATORY FRAMEWORK AND GENERAL INTRODUCTION 1 2. DATA SELECTION FOR CLASSIFICATION 2 2.1. WATER "SOLUBILITY" OF PETROLEUM SUBSTANCES AND TEST "CONCENTRATIONS" 2 2.2. ACUTE AND CHRONIC TOXICITY AND SOLUBILITY LIMITS 2 2.3. PERSISTENCE AND FATE PROCESSES FOR PETROLEUM SUBSTANCES 3 2.4. -

First Telomerisation of Piperylene with Morpholine Using Palladium¬タモ
Catalysis Communications 77 (2016) 70–74 Contents lists available at ScienceDirect Catalysis Communications journal homepage: www.elsevier.com/locate/catcom Short communication First telomerisation of piperylene with morpholine using palladium– carbene catalysts Peter Neubert, Ines Meier, Tom Gaide, René Kuhlmann, Arno Behr ⁎ Technische Universität Dortmund, Lehrstuhl für Technische Chemie, Emil-Figge-Str. 66, 44227 Dortmund, Germany article info abstract Article history: Telomerisation of common 1,3-dienes like butadiene and isoprene has already been successfully conducted with Received 1 July 2015 a huge number of nucleophiles. However, 1,3-pentadiene (piperylene) telomerisation with amines has not been Received in revised form 19 January 2016 reported yet. Here, we present the first telomerisation of piperylene with morpholine, providing an atom eco- Accepted 20 January 2016 nomic access to unsaturated C -amines in a single reaction step. Investigations of the reaction conditions such Available online 25 January 2016 10 as precursor and ligand screening led to Pd(acac)2/IMes·HCl being the most active catalyst. Keywords: © 2016 Elsevier B.V. All rights reserved. Homogeneous catalysis Topic: Telomerisation Dienes Piperylene Amines 1. Introduction with oxygen nucleophiles [22–24]. Nevertheless, piperylene telomerisation has also been achieved with carbon dioxide [25],sul- Having in mind the diminishing resources of fossil feedstocks, one fones [26], and trimethylsilane [27]. To the best of our knowledge, major goal of catalysis is the development of efficient synthesis routes there is no telomerisation of piperylene described with amines. For based on either new feedstocks or on well-known, yet unutilized com- long time, telomerisation reactions have been catalysed by palladium/ pounds. -

Material Safety Data Sheet
Material Safety Data Sheet Piperylene, tech., 60%, cis and trans isomers ACC# 30000 Section 1 - Chemical Product and Company Identification MSDS Name: Piperylene, tech., 60%, cis and trans isomers Catalog Numbers: AC207650000, AC207650010, AC207650500 Synonyms: 1,3-Pentadiene; 1-Methylbutadiene; Penta-1,3-diene; Pentadiene, 1,3- Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 504-60-9 Piperylene 60 207-995-2 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: APHA: 25 max clear liquid. Flash Point: -28 deg C. Danger! Extremely flammable liquid. Causes respiratory tract irritation. Irritant. Lachrymator (substance which increases the flow of tears). Causes eye and skin irritation. May cause digestive tract irritation. May cause central nervous system depression. Target Organs: Central nervous system, respiratory system, eyes, skin. Potential Health Effects Eye: Vapors cause eye irritation. Lachrymator (substance which increases the flow of tears). Causes redness and pain. May cause chemical conjunctivitis and corneal damage. Skin: Causes skin irritation. Causes redness and pain. May cause cyanosis of the extremities. Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Possible aspiration hazard. May cause central nervous system depression. Ingestion of large amounts may cause CNS depression. Inhalation: Causes respiratory tract irritation. May cause effects similar to those described for ingestion. May cause dyspnea (difficult or labored breathing). Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. May cause burning sensation in the chest. -

From Cracker to Key C5 Derivative Applications for Isoprene, DCPD and Piperylene Special Report Prospectus
IHS CHEMICAL C5 Value Chain Study: From Cracker to Key C5 Derivative Applications for Isoprene, DCPD and Piperylene Special Report Prospectus IHS Chemical Prospectus IHS CHEMICAL Contents Contents ..................................................................................................................................... 2 Introduction ................................................................................................................................. 3 What are C5 Petrochemicals? .............................................................................................. 3 What Industries do C5 Petrochemicals Supply? .................................................................. 4 Accessing C5 Petrochemicals .............................................................................................. 6 Issues Impacting the C5 Petrochemicals Industry ................................................................ 8 Study Objective .......................................................................................................................... 9 Study Scope ............................................................................................................................... 9 Market Dynamics for C5 Petrochemicals ............................................................................. 9 Pricing Dynamics for C5 Petrochemicals ............................................................................. 9 Downstream Industries Review ........................................................................................