Animal Genetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SOUTHERN MARYLAND KENNEL CLUB (SUNDAY) Preliminary Entry Breakdown
SOUTHERN MARYLAND KENNEL CLUB (SUNDAY) Preliminary Entry Breakdown BREED DOGS SWEEPS BREAKDOWN MISC D B Barbet 0 ( - ) - ( - ) Brittany 7 ( - ) 1 - 5 ( 1 - 0 ) Lagotto Romagnolo 2 ( - ) 0 - 1 ( 0 - 1 ) Nederlandse Kooikerhondje 0 ( - ) - ( - ) Pointer 11 ( - ) 0 - 6 ( 1 - 4 ) Pointer (German Shorthaired) 12 ( - ) 2 - 3 ( 4 - 3 ) Pointer (German Wirehaired) 3 ( - ) 0 - 0 ( 2 - 1 ) Retriever (Chesapeake Bay) 16 ( - ) 2 - 9 ( 3 - 2 ) Retriever (Curly-Coated) 0 ( - ) - ( - ) Retriever (Flat-Coated) 16 ( - ) 4 - 2 ( 6 - 4 ) Retriever (Golden) 59 ( - ) 20 - 32 ( 5 - 2 ) Retriever (Labrador) 36 ( - ) 16 - 17 ( 0 - 3 ) Retriever (NSDT) 12 ( - ) 1 - 6 ( 2 - 3 ) Setter (English) 3 ( - ) 0 - 1 ( 1 - 1 ) Setter (Gordon) 0 ( - ) - ( - ) Setter (Irish) 17 ( - ) 4 - 9 ( 2 - 2 ) Setter (Irish Red & White) 1 ( - ) 1 - 0 ( 0 - 0 ) Spaniel (American Water) 4 ( - ) 4 - 0 ( 0 - 0 ) Spaniel (Boykin) 4 ( - ) 3 - 1 ( 0 - 0 ) Spaniel (Clumber) 3 ( - ) 1 - 0 ( 1 - 1 ) Spaniel (Cocker) Black 1 ( - ) 0 - 0 ( 1 - 0 ) Spaniel (Cocker) ASCOB 0 ( - ) - ( - ) Spaniel (Cocker) Parti-color 1 ( - ) 0 - 0 ( 1 - 0 ) Spaniel (English Cocker) 13 ( - ) 1 - 8 ( 3 - 1 ) Spaniel (English Springer) 15 ( - ) 3 - 8 ( 3 - 1 ) Spaniel (Field) 2 ( - ) 1 - 0 ( 1 - 0 ) Spaniel (Irish Water) 1 ( - ) 0 - 0 ( 0 - 1 ) Spaniel (Sussex) 4 ( - ) 1 - 2 ( 1 - 0 ) Spaniel (Welsh Springer) 10 ( - ) 2 - 2 ( 4 - 2 ) Spinone Italiano 1 ( - ) 0 - 0 ( 0 - 1 ) Vizsla 32 ( - ) 9 - 12 ( 5 - 6 ) Weimaraner 19 ( - ) 6 - 4 ( 5 - 4 ) Wirehaired Pointing Griffon 4 ( - ) 0 - 0 ( 2 - 2 ) Wirehaired Vizsla 2 ( - -

Dog Breeds Impounded in Fy16
DOG BREEDS IMPOUNDED IN FY16 AFFENPINSCHER 4 AFGHAN HOUND 1 AIREDALE TERR 2 AKITA 21 ALASK KLEE KAI 1 ALASK MALAMUTE 6 AM PIT BULL TER 166 AMER BULLDOG 150 AMER ESKIMO 12 AMER FOXHOUND 12 AMERICAN STAFF 52 ANATOL SHEPHERD 11 AUST CATTLE DOG 47 AUST KELPIE 1 AUST SHEPHERD 35 AUST TERRIER 4 BASENJI 12 BASSET HOUND 21 BEAGLE 107 BELG MALINOIS 21 BERNESE MTN DOG 3 BICHON FRISE 26 BLACK MOUTH CUR 23 BLACK/TAN HOUND 8 BLOODHOUND 8 BLUETICK HOUND 10 BORDER COLLIE 55 BORDER TERRIER 22 BOSTON TERRIER 30 BOXER 183 BOYKIN SPAN 1 BRITTANY 3 BRUSS GRIFFON 10 BULL TERR MIN 1 BULL TERRIER 20 BULLDOG 22 BULLMASTIFF 30 CAIRN TERRIER 55 CANAAN DOG 1 CANE CORSO 3 CATAHOULA 26 CAVALIER SPAN 2 CHESA BAY RETR 1 CHIHUAHUA LH 61 CHIHUAHUA SH 673 CHINESE CRESTED 4 CHINESE SHARPEI 38 CHOW CHOW 93 COCKER SPAN 61 COLLIE ROUGH 6 COLLIE SMOOTH 15 COTON DE TULEAR 2 DACHSHUND LH 8 DACHSHUND MIN 38 DACHSHUND STD 57 DACHSHUND WH 10 DALMATIAN 6 DANDIE DINMONT 1 DOBERMAN PINSCH 47 DOGO ARGENTINO 4 DOGUE DE BORDX 1 ENG BULLDOG 30 ENG COCKER SPAN 1 ENG FOXHOUND 5 ENG POINTER 1 ENG SPRNGR SPAN 2 FIELD SPANIEL 2 FINNISH SPITZ 3 FLAT COAT RETR 1 FOX TERR SMOOTH 10 FOX TERR WIRE 7 GERM SH POINT 11 GERM SHEPHERD 329 GLEN OF IMALL 1 GOLDEN RETR 56 GORDON SETTER 1 GR SWISS MTN 1 GREAT DANE 23 GREAT PYRENEES 6 GREYHOUND 8 HARRIER 7 HAVANESE 7 IBIZAN HOUND 2 IRISH SETTER 2 IRISH TERRIER 3 IRISH WOLFHOUND 1 ITAL GREYHOUND 9 JACK RUSS TERR 97 JAPANESE CHIN 4 JINDO 3 KEESHOND 1 LABRADOR RETR 845 LAKELAND TERR 18 LHASA APSO 61 MALTESE 81 MANCHESTER TERR 11 MASTIFF 37 MIN PINSCHER 81 NEWFOUNDLAND -
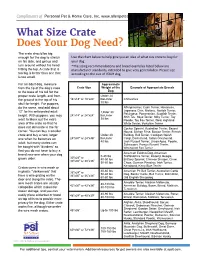
What Size Crate Does Your Dog Need?
Compliments of What Size Crate Does Your Dog Need? The crate should be big enough for the dog to stretch Use the chart below to help give you an idea of what size crate to buy for on his side, and get up and your dog. turn around without his head *The sizing recommendations and breed examples listed below are hitting the top. A crate that is manufacturer standards, intended to give very general idea. Please size too big is better than one that according to the size of YOUR dog. is too small. For an adult dog, measure Approximate from the tip of the dog’s nose Crate Size Weight of the Example of Appropriate Breeds to the base of his tail for the Dog proper crate length, and from Under 24 the ground to the top of his 18"x18" or 18"x24" lbsUnder Chihuahua skull for height. For puppies, 30 lbs do the same, and add about Affenpinscher, Cairn Terrier, Havanese, Japanese Chin, Maltese, Norfolk Terrier, 12” for his anticipated adult Under 30 Pekingese, Pomeranian, Scottish Terrier, 24”x18” or 24”x24” lbsUnder height. With puppies, you may Shih Tzu, Skye Terrier, Silky Terrier, Toy 38 lbs want to block out the extra Poodle, Toy Fox Terrier, West Highland area of the crate so that he White Terrier, Yorkshire Terrier does not eliminate in the far Cocker Spaniel, Australian Terrier, Basset corner. You can buy a smaller Hound, Bichon Frise, Boston Terrier, French crate and buy a new, larger Under 40 Bulldog, Bull Terrier, Cardigan Welsh one when he becomes an 24"x30" or 24"x36" lbsUnder Corgi, Dachshund, Italian Greyhound, adult, but many crates can 40 lbs Jack Russell Terrier, Lhasa Apso, Poodle, Schnauzer, Parson Russell Terrier, be bought with “dividers” so Wirehaired Fox Terrier that you do not have to buy a American Eskimo Dog, American brand new one when your dog 0-40 lbs Staffordshire Terrier, Basenji, Beagle, 30"x24" or grows older. -

TOY FOX TERRIER Official UKC Breed Standard Terrier Group ©Copyright 1936, United Kennel Club Revised July 2011
TOY FOX TERRIER Official UKC Breed Standard Terrier Group ©Copyright 1936, United Kennel Club Revised July 2011 Those dogs appear almost identical to the dogs of today. It was not until February 24, 1936, that U.K.C. began registering the Toy Fox Terrier under its current name. GENERAL APPEARANCE The Toy Fox Terrier is small in size, with a body that is square when viewed from the side. The length of the head, neck and legs are in proportion to the length and depth of the body. The body is compact, with the short tail carried upright. With a short, glossy coat that is predominantly white, the appearance is elegant, balanced and aristocratic. Highly intelligent, alert, loyal, fearless and having much endurance, this small dog, above all, has the conformation, characteristics and personality of a terrier. CHARACTERISTICS The goals and purposes of this breed standard include: The Toy Fox Terrier is self-possessed, spirited and to furnish guidelines for breeders who wish to maintain determined. They are energetic, lively and strong for the quality of their breed and to improve it; to advance their size. They are not easily intimidated by other pets. this breed to a state of similarity throughout the world; Most are comical, entertaining and playful all of their and to act as a guide for judges. life, which is generally long in comparison to many Breeders and judges have the responsibility to avoid other breeds. They are friendly and loyal to their master any conditions or exaggerations that are detrimental to or owners, yet protective. -

South Australian Obedience Dog Club Inc
South Australian Obedience Dog Club Inc Agility Trials Sunday 9th April 2017 SA Obedience Dog Club Agility Trials Sunday 9th April 2017 SOUTH AUSTRALIAN OBEDIENCE DOG CLUB INC Agility Trials Sunday 9th April 2017 At 9.00 am and not before 1.00 pm 9.00 am Brian Fielder ………………. Agility: Master, Open, Excellent, Novice Jane Lawrence ………….. Jumping: Novice, Excellent, Open, Master Not Before 1.00 pm Rose Ince …………... Agility: Master, Open, Excellent, Novice Julie Lyon………….………Jumping: Novice, Excellent, Open, Master SACA Representative: Mr Victor Jordan Trial Manager: Mrs Barbara Richter-Winter Veterinary: By Committee Vetting 8.00 am to 8.45 am and 12.30 to 12.45 pm. Bitches only without desexing certificate or tattoo. All exhibits to pass checkpoint by close. Order of Judging: Two rings simultaneously as above. One course only for all height categories within each class. Prizes: 1st Place qualifying in each height category Sashes: To all qualifiers SA Obedience Dog Club Agility Trials Sunday 9th April 2017 AM Trial Master Agility – Brian Fielder SCT 60 Time T/F C/F Total Rank 300 Class 3001 Inneslake Royal Kiss CD RN ADM JDM JDO SD GD Sally Millan Shetland Sheepdog D/Q 3004 Rex ADX JDX Rosemary Ince Tenterfield Terrier cross D/Q 3005 Donriver Platinum Edition RN ADM JDM GD S & T Millan Shetland Sheepdog 45.11 0 0 0 Q 1st 3006 Asher CD RE ADX JD GD SD SPDX FS.N HTM.N Julie Brown Papillon cross Sheltie D/Q 3009 Ag Ch (300) Ashem Hera ADM7 JDM5 SDX GDX PT Joan Murray Shetland Sheepdog 59.20 0 0 0 Q 2nd 3010 Gumhaven Totally Cool ADX JDX Andrew -

Dog Breed DNA and Survey Results: What Kind of Dog Is That? the Dogs () DNA Results Survey Results
Maddie's Shelter Medicine Program College of Veterinary Medicine (https://sheltermedicine.vetmed.ufl.edu) Dog Breed DNA and Survey Results: What Kind of Dog is That? The Dogs () DNA Results Survey Results Dog 01 Top Responses 25% Toy Fox Terrier Golden Retriever 25% Harrier Pomeranian 15.33% Anatolian Shetland Sheepdog Shepherd Cocker Spaniel 14% Chinese Crested Chihuahua Dog 02 Top Responses 50% Catahoula Leopard Labrador Retriever Dog American Staffordshire 25% Siberian Husky Terrier 9.94% Briard No Predominant Breed 5.07 Airedale Terrier Border Collie Pointer (includes English Pointer) Dog 03 Top Responses 25% American Labrador Retriever Staffordshire German Shepherd Dog 25% German Shepherd Rhodesian Ridgeback 25% Lhasa Apso No Predominant Breed 25% Dandie Dinmont Terrier American Staffordshire Terrier Dog 04 Top Responses 25% Border Collie Wheaten Terrier, Soft Coated 25% Tibetan Spaniel Bearded Collie 12.02% Catahoula Leopard Dog Briard 9.28% Shiba Inu Cairn Terrier Tibetan Terrier Dog 05 Top Responses 25% Miniature Pinscher Australian Cattle Dog 25% Great Pyrenees German Shorthaired Pointer 10.79% Afghan Hound Pointer (includes English 10.09% Nova Scotia Duck Pointer) Tolling Retriever Border Collie No Predominant Breed Dog 06 Top Responses 50% American Foxhound Beagle 50% Beagle Foxhound (including American, English, Treeing Walker Coonhound) Harrier Black and Tan Coonhound Pointer (includes English Pointer) Dog 07 Top Responses 25% Irish Water Spaniel Labrador Retriever 25% Siberian Husky American Staffordshire Terrier 25% Boston -

Official UKC Terrier Race Rulebook
UNITED KENNEL CLUB, INC. • 100 E Kilgore Rd • Kalamazoo, MI 49002-5584 (269) 343-9020 • www.ukcdogs.com • [email protected] Official UKC Terrier Race Rulebook Regulations Governing UKC® Licensed Terrier Races Effective January 1, 2014 Changes are indicated by bold, italic font. Table of Contents Section I. Jurisdiction..............................................1 Section XVI. Non-Licensed Terrier Race Classes....10 Section II. Who may offer terrier races..................1 Section XVII. Race course requirements................10 Section III. Definitions ..........................................1 Section XVIII. Equipment Requirements...................11 Section IV. General Rules .................................... 2 Section XIX. Racing Procedure............................12 Section V. Eligible Breeds .................................... 3 Section XX. Awards, trophies, ribbons and Section VI. Entering a UKC event ........................ 3 placements....................................................13 Section VII. Judging Schedule............................... 4 Section XXI. Judging Procedures ..........................13 Section VIII. Judge Changes................................. 4 Section XXII. Terrier Race Judge Information..........14 Section IX. Terrier Race Handlers......................... 4 Section XXIII. Dog Temperament and Behavior....16 Section X. Rules applying to exhibitors and Section XXIV. Use of Alcohol and Illegal Drugs spectators....................................................... 4 at Events ..........................................................16 -

Full Competitor List
Competitor List Argentina Entries: 2 Height Handler Dog Breed 300 Fernando Estevez Costas Arya Mini Poodle 600 Jorge Esteban Ramos Trice Border Collie Salinas Australia Entries: 6 Height Handler Dog Breed 400 Reserve Dog Casper Dutch Smoushond 500 Maria Thiry Tebbie Border Collie 500 Maria Thiry Beat Pumi 600 Emily Abrahams Loki Border Collie 600 Reserve Dog Ferno Border Collie 600 Gillian Self Showtime Border Collie Austria Entries: 12 Height Handler Dog Breed 300 Helgar Blum Tim Parson Russell Ter 300 Markus Fuska Cerry Lee Shetland Sheepdog 300 Markus Fuska Nala Crossbreed 300 Petra Reichetzer Pixxel Shetland Sheepdog 400 Gabriele Breitenseher Aileen Shetland Sheepdog 400 Gregor Lindtner Asrael Shetland Sheepdog 400 Daniela Lubei Ebby Shetland Sheepdog 500 Petra Reichetzer Paisley Border Collie 500 Hermann Schauhuber Haily Border Collie 600 Michaela Melcher Treat Border Collie 600 Sonja Mladek Flynn Border Collie 600 Sonja Mladek Trigger Border Collie Belgium Entries: 17 Height Handler Dog Breed 300 Dorothy Capeta Mauricio Ben Jack Russell Terrier 300 Ann Herreman Lance Shetland Sheepdog 300 Brechje Pots Dille Jack Russell Terrier 300 Kristel Van Den Eynde Mini Jack Russell Terrier 400 Ann Herreman Qiell Shetland Sheepdog 400 Anneleen Holluyn Hjatho Mini Australian Shep 400 Anneleen Holluyn Ippy Mini Australian Shep 26-Apr-19 19:05 World Agility Open 2019 Page 1 of 14 500 Franky De Witte Laser Border Collie 500 Franky De Witte Idol Border Collie 500 Erik Denecker Tara Border Collie 500 Philippe Dubois Jessy Border Collie 600 Kevin Baert -

Class ID Call Name Breed ID First Name Last Name
Class ID Call Name Breed ID First Name Last Name Score Time Place Ring 1 Ratters 0208T1LRM Nessa Windsprite Andrea Rogers 100 26.41 0208T1LRA Whit Border Collie HR649 Barb Black 100 38.6 0208T1LRA Marty Golden Retriever HR674 Kayleigh Davenport 90 61.93 0208T1LRM Maggie May Windsprite HR658 Patricia Stogryn 100 39.81 ratters 2 0208T2LRM Nessa Windsprite Andrea Rogers 90 95.47 1 0208T2LRA Marty Golden Retriever HR674 Kayleigh Davenport 100 55.44 0208T2LRA Whit Border Collie HR649 Barb Black 100 35.56 0208T2LRM Maggie May Windsprite HR658 Patricia Stogryn 20 150 2 Happy Ratters 1/2 0208T1LHM Penny All American HR019 Nichole Burke abs 0208T2LHM Penny All American HR019 Nichole Burke abs Expert Beetle Beagle HR004 Andrea Rogers 90 153.31 1 Beetle Beagle HR004 Andrea Rogers 90 174.15 1 Champion 0208T1LCA Willow Beagle HR062 Cheryl Hill abs 0208T1LCA Mikey Dalmatian HR128 Jody Fraser 100 135.22 1 0208T1LCM Trax Rat Terrier HR419 Barb Black 80 240 1 0208T1LCM Harley Beagle HR005 Cheryl Hill abs 2 0208T2LCA Willow Beagle HR062 Cheryl Hill abs 0208T2LCA Mikey Dalmatian HR128 Jody Fraser 90 230.66 0208T2LCM Trax Rat Terrier HR419 Barb Black 60 240 0208T2LCM Harley Beagle HR005 Cheryl Hill abs Rapid Rat Relay 0208T1LSA Trax Rat Terrier HR419 Barb Black 50 130.35 2 0208T1LSM Nessie AmStaff HR115 Jen Belanger 110 156.62 2 0208T1LSA Fallon Smooth Fox Terrier HR035 Bonnie Bartlett 110 89.87 1 0208T1LSM Arthur Beagle Mix HR325 Elaine Loranz 75 115.28 3 0208T1LSM Roxy Min. Aussie HR028 Nancy Webb 110 93.53 1 Class ID Call Name Breed ID First Name Last Name Ring 2 Infestation 0208T1LIM Fae Patterdale Terrier HR165 Pauline Goodwin 150 175.03 0208T1LIA Willow Beagle HR062 Cheryl Hill abs 0208T1LIA Whit Border Collie HR649 Barb Black 75 186.75 0208T1LIM Nessie AmStaff HR115 Jen Belanger 150 123.13 3 0208T1LIM Roxy Min. -
Reg Call Name Trialno Class Level Breed Height Owner Result Time
Revolution Dog Sports Gavilan Kennel Club 05/04/19 - 05/05/19 Marked Catalog Reg Call Name TrialNo Class Level Breed Height Owner Result Time/ScoPlace BH-26769 Montey Trial 1 Crazy 8s Patterdale Terrier M David ThompsonP40 BH-32534 Mystic Trial 1 Crazy 8s Beauceron L Robin Gowen P 40 BH-30244 Hammer Trial 1 Crazy 8s Labrador Retriever L Jen Bailey P 50 Reg Call Name TrialNo Class Level Breed Height Owner Result Time/ScoPlace BH-38172 ZOE Trial 1 Instinct Miniature Schnauzer M Claudian Tessan F BH-38192 Zoe Trial 1 Instinct Miniature Schnauzer M Marie Kidwell F BH-38877 Camelot Trial 1 Instinct Samoyed L Julianne GendraP 00:17.8 BH-38693 Eva Trial 1 Instinct Cavalier King Charles SpM Mary Hutchings P 00:43.9 Reg Call Name TrialNo Class Level Breed Height Owner Result Time/ScoPlace BH-21133 Blade Trial 1 Master A Parson Russell Terrier S Liz Carter F BH-13834 Gus Trial 1 Master B Border Terrier M Julie Pryce F BH-26769 Montey Trial 1 Master B Patterdale Terrier M David ThompsonF BH-03940 Zara Trial 1 Master B Weimaraner L Gillian Norris F BH-20954 Isaac Trial 1 Master B German Shepherd Dog L Marianne Laouri F BH-30244 Hammer Trial 1 Master B Labrador Retriever L Jen Bailey P 03:15.3 Reg Call Name TrialNo Class Level Breed Height Owner Result Time/ScoPlace BH-28336 Kacie Trial 1 Novice B Redbone Coonhound L Taylor Santo F BH-24336 Maizie Trial 1 Novice B Poodle, Standard L Elisabeth DeSim F BH-35763 Shakira Trial 1 Novice A Doberman Pinscher L Yvonne Torrez F BH-38877 Camelot Trial 1 Novice A Samoyed L Julianne GendraF BH-37335 Tsuki Trial -

Australian National Kennel Council Limited National Animal Registration Analysis 2010-2019
AUSTRALIAN NATIONAL KENNEL COUNCIL LIMITED NATIONAL ANIMAL REGISTRATION ANALYSIS 2010-2019 GROUP 1 TOYS 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 Affenpinscher 17 33 28 51 30 27 25 Australian Silky Terrier 273 277 253 237 199 202 134 Bichon Frise 445 412 445 366 471 465 465 Cavalier King Charles Spaniel 2942 2794 2655 2531 2615 2439 2555 Chihuahua (Long) 690 697 581 565 563 545 520 Chihuahua (Smooth) 778 812 724 713 735 789 771 Chinese Crested Dog 286 248 266 216 221 234 207 Coton De Tulear 0 0 0 0 0 0 2 English Toy Terrier (Black & Tan) 72 61 54 92 58 63 75 Griffon Bruxellois 199 149 166 195 126 166 209 Havanese 158 197 263 320 340 375 415 Italian Greyhound 400 377 342 354 530 521 486 Japanese Chin 66 43 55 61 45 50 53 King Charles Spaniel 29 11 27 29 24 47 25 Lowchen 53 44 67 84 76 59 60 Maltese 315 299 305 296 234 196 185 Miniature Pinscher 209 217 179 227 211 230 195 Papillon 420 415 388 366 386 387 420 Pekingese 186 161 153 172 196 160 173 Pomeranian 561 506 468 410 428 470 471 Pug 1495 1338 1356 1319 1311 1428 1378 Russian Toy 0 0 3 23 37 22 32 Tibetan Spaniel 303 210 261 194 220 226 183 Yorkshire Terrier 237 253 168 234 188 201 227 TOTAL 10134 9554 9207 9055 9244 9302 9266 0 0 0 Page 1 AUSTRALIAN NATIONAL KENNEL COUNCIL LIMITED NATIONAL ANIMAL REGISTRATION ANALYSIS 2010-2019 GROUP 2 TERRIERS 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 Airedale Terrier 269 203 214 235 308 281 292 American Hairless Terrier 0 0 0 0 0 0 0 American Staffordshire Terrier 1625 2015 1786 2337 2112 2194 1793 Australian Terrier 272 247 308 -

Dogs BFA No. Dogs Name Jump Height Breed 0061K Wizz 11 BC
Dogs BFA Dogs Name Jump Breed No. height 0061K Wizz 11 BC/WSD 0062K Pippa 7 Crossbreed 0062L Fly 9 Crossbreed 0062O Ronnie 10 Border Collie/WSD 0080L Apple Pie 11 Cross-Breed 0111K Sid 9 Crossbreed 0157C Jamie 8 Crossbreed 0203D Tigs 8 JRT 0204G Billie Boy 11 Crossbreed 0204J Jazz 9 Cross-Breed 0217D Tilly 8 Crossbreed 0277I Ted 7 JRT 0311B Alfie 11 Bedlington Terrier 0337C Millie 9 Crossbreed 0366C Shale 7 Patterdale 0381C Kaos 11 ECS 0412H Uhu 9 ECS 0412L Bumble 11 Border Collie/WSD 0412M Beebee 7 JRT 0423B Yogi 10 English Cocker Spaniel 0473F Saffy 11 BC/WSD 0524E Rosie 10 Cross-Breed 0540J Raffy 7 JRT 0540M Delta 8 English Cocker Spaniel 0556E Kiki 10 Spanish Water Dog 0557E Sol 11 Spanish Water Dog 0668F Asbo 10 Cross-Breed 0693D Roxy 7 JRT 0693F Arnie 7 Jack Russell Terrier 0703H Apache 9 Cross-Breed 0719D Lacey Nikkas 9 Crossbreed 0719E Pascoe 11 ECS 0753A Willow 11 BC/WSD 0783B Ruby 8 Crossbreed 0827A Fraggle 10 Crossbreed 0828A Annie 8 Crossbreed 0909C Kai 7 JRT 0980D Cassie 11 Border Collie/WSD 1068C Ellie 8 Miniature American Shepherd 1077C Mack 7 JRT 1112I Taz 8 ECS 1119C Mary Doll 7 Crossbreed 1122E Gem 9 Cross-Breed 1135L Popeye 11 Cross-Breed 1148F Wacky Jacky 7 JRT 1254C Millie 9 ECS 1254F Milo 11 ESS 1255C Daisy May 7 Crossbreed 1277D Dante 10 ECS 1277G Barley 9 Crossbreed 1285M Fluke 11 Crossbreed 1285Q Tohrment 10 Cross-Breed 1324C Arlo 8 ECS 1365A Rolo 11 Crossbreed 1387C Ace 9 Cross-Breed 1423E Sarge 9 Staffordshire Bull Terrier 1430D Fidgit 11 English Cocker Spaniel 1443F Forest 10 Crossbreed 1443I Ollie 9 Crossbreed