Imperial College London Soil Microbial Interactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MS Annual Report Dec 05.Qxd
December 2005 Parliamentary News Martin Salter’s Eighth Annual Report to Constituents Reading West Constituency – which includes: Pangbourne, Purley, Tilehurst, Theale, Calcot, Coley, Norcot, Southcote, Whitley and the Oxford Road Working for Reading West Since he was first elected as Member of Parliament in 1997 Martin Salter has established a reputation as a hard-working constituency MP who gets results for local people. Martin believes in keeping in touch with his constituents and produces an Annual Report setting out what he has been doing on your behalf during the last year. “ Dear Resident 2005 has been a year of triumphs and disasters. It began with the awful scenes of devastation from the Asian Tsunami which prompted a generous worldwide response in which the people of Reading played their part. We scarcely had time to enjoy the triumph of Britain winning the Olympics bid before the dreadful London bombings of 7th July. COMMUNITY CHAMPIONS – The Prime Minister asked Martin to invite two community workers to 10 Downing Street. Street warden Ahmed Abd-Eighany Obviously I was pleased to have been re-elected for the and Battle Library’s Marjorie McClure were thanked personally by Tony Blair for their hard work. third time in a row and in the constituency I’ve been as busy as ever: successfully fighting off plans to merge Calcot Infant and Junior Schools; winning extra funding for Salter Wins a Third Term Prospect College, Denefield, Brookfields and Long Lane Reading West MP, Martin Salter, was comfortably re- Primary Schools; helping get rid of the illegal travellers elected on May 5th to serve a third term in Parliament. -
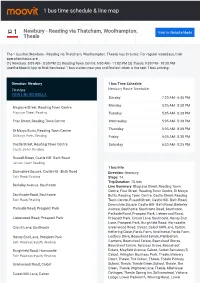
1 Bus Time Schedule & Line Route
1 bus time schedule & line map 1 Newbury - Reading via Thatcham, Woolhampton, View In Website Mode Theale The 1 bus line (Newbury - Reading via Thatcham, Woolhampton, Theale) has 3 routes. For regular weekdays, their operation hours are: (1) Newbury: 5:05 AM - 8:30 PM (2) Reading Town Centre: 5:00 AM - 11:02 PM (3) Theale: 9:30 PM - 10:30 PM Use the Moovit App to ƒnd the closest 1 bus station near you and ƒnd out when is the next 1 bus arriving. Direction: Newbury 1 bus Time Schedule 74 stops Newbury Route Timetable: VIEW LINE SCHEDULE Sunday 7:20 AM - 6:45 PM Monday 5:05 AM - 8:30 PM Blagrave Street, Reading Town Centre Blagrave Street, Reading Tuesday 5:05 AM - 8:30 PM Friar Street, Reading Town Centre Wednesday 5:05 AM - 8:30 PM St Marys Butts, Reading Town Centre Thursday 5:05 AM - 8:30 PM St Mary's Butts, Reading Friday 5:05 AM - 8:30 PM Castle Street, Reading Town Centre Saturday 6:20 AM - 8:25 PM Castle Street, Reading Russell Street, Castle Hill - Bath Road Janson Court, Reading 1 bus Info Downshire Square, Castle Hill - Bath Road Direction: Newbury Bath Road, Reading Stops: 74 Trip Duration: 78 min Berkeley Avenue, Southcote Line Summary: Blagrave Street, Reading Town Centre, Friar Street, Reading Town Centre, St Marys Southcote Road, Southcote Butts, Reading Town Centre, Castle Street, Reading Bath Road, Reading Town Centre, Russell Street, Castle Hill - Bath Road, Downshire Square, Castle Hill - Bath Road, Berkeley Parkside Road, Prospect Park Avenue, Southcote, Southcote Road, Southcote, Parkside Road, Prospect Park, Liebenrood -

86 Bus Time Schedule & Line Route
86 bus time schedule & line map 86 Calcot Mill Lane - Little Heath School via Meadway View In Website Mode Precinct The 86 bus line (Calcot Mill Lane - Little Heath School via Meadway Precinct) has 2 routes. For regular weekdays, their operation hours are: (1) Calcot: 3:35 PM (2) Little Heath: 8:08 AM Use the Moovit App to ƒnd the closest 86 bus station near you and ƒnd out when is the next 86 bus arriving. Direction: Calcot 86 bus Time Schedule 29 stops Calcot Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday Not Operational Little Heath School, Little Heath Tuesday 3:35 PM Hallplace Farm, Little Heath Wednesday 3:35 PM Chapel Hill Westwood Glen, Tilehurst Thursday 3:35 PM Chapel Hill, Reading Friday Not Operational Normanstead Road, Tilehurst Saturday Not Operational Chapel Hill Lower Elmstone Drive, Tilehurst Chapel Hill, Tilehurst Lansdowne Road, Tilehurst 86 bus Info Direction: Calcot Halls Road, Tilehurst Stops: 29 Trip Duration: 27 min Line Summary: Little Heath School, Little Heath, St Michaels, Tilehurst Hallplace Farm, Little Heath, Chapel Hill Westwood Hollicombe Road, Reading Glen, Tilehurst, Normanstead Road, Tilehurst, Chapel Hill Lower Elmstone Drive, Tilehurst, Chapel Hill, Corwen Road, Tilehurst Tilehurst, Lansdowne Road, Tilehurst, Halls Road, Tilehurst, St Michaels, Tilehurst, Corwen Road, Church End Lane, Meadway Tilehurst, Church End Lane, Meadway, Dryland House, Meadway, Usk Road, Meadway, Stoneham Dryland House, Meadway Close Footpath, Meadway, Meadway Precinct, Meadway, Prospect College, Prospect -

James Field, Violet Lane FPS/J1155/7/59, and Schedule 14 Application NATROW/J1155/529A/08/90 Made 3Rd July 2008 Page 0 of 34
James Field, Violet Lane FPS/J1155/7/59, and Schedule 14 Application NATROW/J1155/529A/08/90 made 3rd July 2008 Page 0 of 34 1 Page 0 of 34 DL666 East Park : Five Fords Barton (Farm), a working farm of heritage and culture - versus - Modification Order 2006 Content-Publishing-Copyright-restricted, not for public sale, performance or display; limited to direct usage in Definitive Map Review Uffculme only unless prior permission granted. Publication rights reserved James Field or his heirs. To be printed in colour only, no BW James Field, Violet Lane FPS/J1155/7/59, and Schedule 14 Application NATROW/J1155/529A/08/90 made 3rd July 2008 Page 1 of 34 2 James Field 2nd October 2012 This document should be reproduced in colour, any black & white copying will be 3 misconstrued, & deemed tampering with ‘Evidence’. It represents that delivered to the Planning Inspectorate 29th June 2012 4 Rights of Way Section Modification Order Inquiry Madgelake Hall , Uffculme [Handed in on 3rd October 2012] 5 Planning Inspectorate 6 Temple Quay House This document contains both minor changes, & additions inclusive of legal maxims and 7 2 The Square precedence, to improve, & further the reader’s ability to comprehend the document & the 8 Bristol beneficial information contained within regarding inquiry fixing and public office fraud. 9 BS1 6PN 10 With reference to the Devon Green Lanes Schedule 14 Application FPS/J1155/7/98 : [NATROW/J1155/529A/08/90] made 11 3rd July 2008, to add a further footpath across Five Fords Farm, Uffculme. This current Modification Order involves the same 12 location and people, and evidence relied on as that used during expropriation of Violet Lane during two sham Public 13 Inquiries: 18th Sept 2007, and 15th July 2008. -

Directory of Services and Activities for People Living with Dementia
Directory of services and activities for people living with dementia The aim of this directory is to provide information about activities that are available locally for people living with dementia so that they are able to participate within their communities. Yoga Activities Relaxed seated exercise for all abilities Thursdays 2-3.30pm Central (Newbury) Thatcham Rugby Club, Henwick Lane, Singing for the Brain RG18 3BN Mondays 10.30am-12pm Phone to confirm dates available Riverside Centre, Rosemoor Gardens, Clay Hill, Phone no: 0118 959 6482 Newbury RG14 2FG Email: [email protected] Email: [email protected] Phone no: 0118 959 6482 Memories café Last Friday of every month 10.30am – Chair Exercises 12.30pm in the coffee shop at Winchcombe Mondays 10am Place Care Home, Maple Fair Close Centre, Newtown Road, Crescent, Newbury, Berkshire, RG14 1LN Newbury RG14 7BH Phone no: Claire Parsons 01635 275400 Email: [email protected] Email: [email protected] Phone no: 01635 40488/41294 Activity Centre Bingo Audrey Needham House, Tuesday and Fridays 2pm 29 Victoria Grove, Newbury, RG14 7RB Fair Close Centre, Newtown Road, Wednesdays 9.30 – 4pm Newbury RG14 7BH Full day of activities, with care and support, a Email: [email protected] two-course lunch and refreshments Phone no: 01635 40488/41294 Phone no: 0118 950 7914 or email: [email protected] Knit and Natter Wednesdays 1pm Fair Close Centre, Newtown Road, Relaxed Theatre Performances - Newbury RG14 7BH The Newbury Corn Exchange Email: [email protected] Contact theatre for information on performances Phone no: 01635 40488/41294 Newbury Corn Exchange, Market Place, Newbury RG14 5BD Poetry Group Email: [email protected] Every other month from 12.30pm Phone no: 01635 582666 Berkshire Arms, Midgham, RG7 5UK Phone no: 0118 959 6482 Relaxed Theatre Performances - Email: [email protected] The Watermill Theatre Contact theatre for information on performances. -

HOLYBROOK PARISH NEWS Serving the Communities of Fords Farm and Beansheaf Farm
HOLYBROOK PARISH NEWS Serving the communities of Fords Farm and Beansheaf Farm HOLYBROOKOctober / November 2018 PARISH NEWS www.holybrookparishcouncil.co.uk/[email protected] Serving the communities of Fords Farm and Beansheaf Farm October / November 2018 www.holybrookparishcouncil.co.uk/[email protected] Dear Residents, We hope you have had a peaceful summer and are enjoying the beautiful autumn months. West Berkshire Council have made changes to the way they address litter and, sadly, we have seen an increase of litter within the Parish. Therefore, your Parish Councillors will be organising Councillor lead community litter picks over the coming year. Dates to follow. A ‘SPECIAL EDITION’ newsletter will arrive with you shortly containing information on the Community Infrastructure Levy survey which was conducted earlier this year. Please keep an eye out for it. As always, if you have any Parish related issues please contact the Parish Office. The local Police Neighbourhood Team Parish Meetings: have been working closely with residents For an up-to-date list of all Parish in Calcot to protect a vulnerable female who was being exploited in meetings, including sub-committees, her home by unwanted persons frequenting her address. This not please visit: only caused problems for the female but also associated Anti-Social Behaviour for the neighbours. www.holybrookparishcouncil.co.uk We thank the residents for their support in providing evidence which Holybrook Parish Council is on we were able to take to Reading Magistrates Court and secure a Facebook & Twitter. Please LIKE our partial house closure order. This means that the Police will continue pages to receive updates and to carry out unannounced checks at the address and anyone found information on what is happening in inside (apart from the female who lives there) is subject to arrest your Parish. -

Winter06.Pub (Read-Only)
WINTER EDITION HOLYBROOK DECEMBER 2006 Inside this issue: Parish Plan Projects 1 .A MESSAGE FROM P A R I S H P L A N UNDERWOOD Underwood Road 1 THE CHAIRMAN PROJECTS ROAD ACTION Action Group GROUP (URAG) The past few months have been very busy for councillors and Following the publication of the Neighbourhood Action 2 the parish office staff as we Parish Plan, an Underwood Road Group have spent many hours dealing Action Group (URAG) has been with the Kennet Valley Park Several projects are established, comprising councillors development proposals, as well now under way, not and parishioners, to consult with as dealing with ordinary council least the Holybrook the community and lend weight to Neighbourhood 2 West Berkshire Council’s efforts to business and matters arising Festival to be held 2nd force the owners of the Wardens from the Parish Plan. At the June 2007. This is a Underwood Road precinct to time of writing we anticipate a real opportunity for redevelop the site. meeting of the West Berkshire Council’s Eastern Area Planning community groups and Linear Park 2 Committee on 15 November organisations to get At a meeting in September the to discuss the Prudential’s involved, raise funds group drew up a ‘wish list’ and proposals. Local councils and and get recognised! plans for further consultation, groups are united in opposition and by the time this newsletter and we hope the result of West is published it will have met Road Safety 3 Berkshire’s meeting will be on again. Please look at the Tick Tock Toy Library We do need a large the council website by the time council website for further Women’s Institute this newsletter appears. -
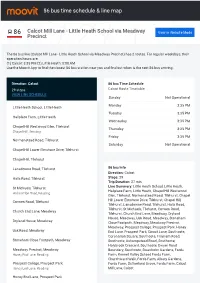
86 Bus Time Schedule & Line Route
86 bus time schedule & line map 86 Calcot Mill Lane - Little Heath School via Meadway View In Website Mode Precinct The 86 bus line (Calcot Mill Lane - Little Heath School via Meadway Precinct) has 2 routes. For regular weekdays, their operation hours are: (1) Calcot: 3:35 PM (2) Little Heath: 8:08 AM Use the Moovit App to ƒnd the closest 86 bus station near you and ƒnd out when is the next 86 bus arriving. Direction: Calcot 86 bus Time Schedule 29 stops Calcot Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday 3:35 PM Little Heath School, Little Heath Tuesday 3:35 PM Hallplace Farm, Little Heath Wednesday 3:35 PM Chapel Hill Westwood Glen, Tilehurst Thursday 3:35 PM Chapel Hill, Reading Friday 3:35 PM Normanstead Road, Tilehurst Saturday Not Operational Chapel Hill Lower Elmstone Drive, Tilehurst Chapel Hill, Tilehurst Lansdowne Road, Tilehurst 86 bus Info Direction: Calcot Halls Road, Tilehurst Stops: 29 Trip Duration: 27 min Line Summary: Little Heath School, Little Heath, St Michaels, Tilehurst Hallplace Farm, Little Heath, Chapel Hill Westwood Hollicombe Road, Reading Glen, Tilehurst, Normanstead Road, Tilehurst, Chapel Hill Lower Elmstone Drive, Tilehurst, Chapel Hill, Corwen Road, Tilehurst Tilehurst, Lansdowne Road, Tilehurst, Halls Road, Tilehurst, St Michaels, Tilehurst, Corwen Road, Church End Lane, Meadway Tilehurst, Church End Lane, Meadway, Dryland House, Meadway, Usk Road, Meadway, Stoneham Dryland House, Meadway Close Footpath, Meadway, Meadway Precinct, Meadway, Prospect College, Prospect Park, Honey Usk -

Abbot Cook, London Road, Originally Named the Jack of Both Sides
Abbot Cook, London Road, originally named the Jack of Both Sides. Joseph Pickett was licensee in 1841 and remained so till around 1865 when Edwin Moss took over. In one directory Moss is described as a fly proprietor, livery stable keeper and victualler. After a brief tenure by John Loveday, Joseph Watts was in charge during the 1880s but by 1891 the licensee was George Talbot, then aged 33. George ran the pub for many years, latterly assisted by his daughter, Mary, as barmaid, and then in or before 1911 his third son, Herbert Lawrence Talbot took over, remaining landlord for the best part of 30 years. A ‘German band’ used to play here in 1913, ‘one outside the pub and two inside’. The stability provided by the Talbot dynasty was quite in contrast to the post-war period when changes of licensee became much more frequent and the pub gradually acquired a less pleasant reputation in which the supply of illegal substances played a part. It was described in Reading Between the Lines (1984- 5) as ‘usually in a state of being boycotted owing to the type of landlord Courage insist on placing there’. It was taken over in the late 1990s by the It’s a Scream brand and renamed Upin Arms; the punning did not stop at the pub sign but included the designation of the toilets. Following a change in the policy of the Mitchell and Butlers pub group it was renamed Abbot Cook in 2010 with a new emphasis on real ale and food. This latest appellation is a reference to Hugh Faringdon Cook, the last Abbot of Reading, who was executed for high treason in 1539 by his erstwhile friend, King Henry VIII and who also has a Roman Catholic secondary school to his name in the Southcote district of Reading. -

87 Bus Time Schedule & Line Route
87 bus time schedule & line map 87 Reading Station - Little Heath School via Southcote, View In Website Mode Ford's Farm, Beansheaf The 87 bus line (Reading Station - Little Heath School via Southcote, Ford's Farm, Beansheaf) has 2 routes. For regular weekdays, their operation hours are: (1) Little Heath: 7:36 AM (2) Reading Town Centre: 3:35 PM Use the Moovit App to ƒnd the closest 87 bus station near you and ƒnd out when is the next 87 bus arriving. Direction: Little Heath 87 bus Time Schedule 46 stops Little Heath Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday 7:36 AM Blagrave Street, Reading Town Centre Railair coach road, Reading Tuesday 7:36 AM Friar Street, Reading Town Centre Wednesday 7:36 AM St Marys Butts, Reading Town Centre Thursday 7:36 AM St Mary's Butts, Reading Friday 7:36 AM Castle Street, Reading Town Centre Saturday Not Operational Castle Street, Reading Russell Street, Castle Hill - Bath Road Janson Court, Reading 87 bus Info Downshire Square, Castle Hill - Bath Road Direction: Little Heath Bath Road, Reading Stops: 46 Trip Duration: 49 min Berkeley Avenue, Southcote Line Summary: Blagrave Street, Reading Town Centre, Friar Street, Reading Town Centre, St Marys Southcote Road, Southcote Butts, Reading Town Centre, Castle Street, Reading Bath Road, Reading Town Centre, Russell Street, Castle Hill - Bath Road, Downshire Square, Castle Hill - Bath Road, Berkeley Monks Way, Southcote Avenue, Southcote, Southcote Road, Southcote, Monks Way, Southcote, Southcote Farm Lane, Southcote Farm Lane, Southcote -

Busmap July2014 Reading Buses Busmap.Qxd 10/06/2014 12:31 Page 1
BusMap_July2014_Reading Buses_BusMap.qxd 10/06/2014 12:31 Page 1 index to places served by bus route information Service frequencies (approx) destination map bus routes service & route Mon - Fri Mon - Fri & evenings & ref. operator rush hours Sat daytimes Sundays Amersham Road J4 27, 29 Premier routes in Greater Reading ASDA Lower Earley L9 19b, 21, N21 ASDA Tilehurst E6 33 5 RB Station - Northumberland Avenue 8 mins 10-12 mins 20-30 mins 6, 6a RB Station - Basingstoke Road - Whitley Wood (6a to Tesco Distribution Centre) 10 mins 10-12 mins half hourly Bath Road E7 1, 2, 2a, 28/28a 9 RB Station - Royal Berks Hospital (RBH) - Shinfield Park 10-15 mins 10-20 mins half hourly (Sundays) hourly (evenings) Beansheaf B8 26, N26 11 RB Coley Park - Central Reading 20 mins 20 mins half hourly (Sundays) hourly (evenings) Bennet Road I9 6, 6a, 72, 82, 82k, N5 13 RB Station - Bulmershe - Woodley Centre - Woodley Airfield approx 30 mins 30 mins hourly Bulmershe M6 13, 19a, 19c 14 RB Station - Shepherds Hill - Woodley Centre - Colemans Moor approx 30 mins 30 mins hourly (evenings) up to 3 per hour (Sundays) Calcot A8 1, 18, 26, N26 15 RB Station - Oxford Road - Dee Park - Tilehurst Triangle 30 mins 30 mins half hourly to Dee Park Caversham Centre H4 22, 24, 25, 27, 28/28a, X39/40, 800, N23 16 RB Station - Oxford Road - Kentwood - Overdown Road - Purley 15 mins 15 mins half hourly Caversham Heights G3 22, X39, X40 allDayallNight 17 RB Tilehurst Water Tower - Central Reading - Wokingham Road 7-8 mins 10 mins 10-20 mins and hourly all night Caversham Park -
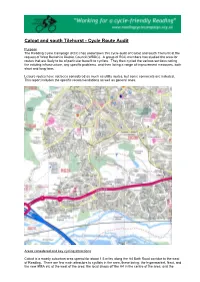
Calcot and South Tilehurst - Cycle Route Audit
Calcot and south Tilehurst - Cycle Route Audit Purpose The Reading Cycle Campaign (RCC) has undertaken this cycle audit of Calcot and south Tilehurst at the request of West Berkshire District Council (WBDC). A group of RCC members has studied the area for routes that are likely to be of particular benefit to cyclists. They then cycled the various sections noting the existing infrastructure, any specific problems, and then listing a range of improvement measures, both short and long term. Leisure routes have not been considered as much as utility routes, but some comments are included. This report includes the specific recommendations as well as general ones. Areas considered and key cycling attractions Calcot is a mainly suburban area spread for about 1.5 miles along the A4 Bath Road corridor to the west of Reading. There are few main attractors to cyclists in the area, these being: the hypermarket, Next, and the new IKEA etc at the west of the area; the local shops off the A4 in the centre of the area; and the Kennet Valley Community Centre and Primary School at the eastern boundary. Other attractors are likely to be Reading town centre about three miles to the east, Theale School one mile to the west, Little Heath School to the north of the A4, and Prospect School half a mile to the east of the Calcot area. Cycling into Reading may be a quite feasible commute. Taking an average cycling speed of 10mph, this gives a time to cycle into central Reading of about 20-30 minutes, which is competitive to travel by car and bus journeys at peak times.