Prokaryotic Cells: Structural Organisation of the Cytoskeleton and Organelles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
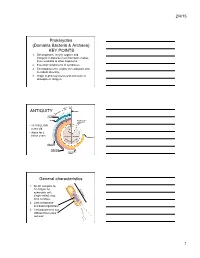
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

The Bacterial Cytoskeleton: More Than Twisted Filaments
NIH Public Access Author Manuscript Curr Opin Cell Biol. Author manuscript; available in PMC 2014 February 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Curr Opin Cell Biol. 2013 February ; 25(1): 125–133. doi:10.1016/j.ceb.2012.10.019. The bacterial cytoskeleton: more than twisted filaments Martin Pilhofer* and Grant J. Jensen Howard Hughes Medical Institute and Division of Biology, California Institute of Technology, 1200 E California Blvd, M/C 114-96, Pasadena, CA, USA Abstract Far from being simple “bags” of enzymes, bacteria are richly endowed with ultrastructures that challenge and expand standard definitions of the cytoskeleton. Here we review rods, rings, twisted pairs, tubes, sheets, spirals, moving patches, meshes and composites, and suggest defining the term “bacterial cytoskeleton” as all cytoplasmic protein filaments and their superstructures that move or scaffold (stabilize/position/recruit) other cellular materials. The evolution of each superstructure has been driven by specific functional requirements. As a result, while homologous proteins with different functions have evolved to form surprisingly divergent superstructures, those of unrelated proteins with similar functions have converged. Defining the bacterial cytoskeleton The word “skeleton” is defined as the basic frame or supporting structure of an object. The term “cytoskeleton” was coined after a network of long, skinny, cell-shape-determining structures was discovered in the cytoplasm of eukaryotic cells. These structures were later found to consist of actin, tubulin and intermediate filament (IF) proteins that move objects through their own growth and disassembly, act as stationary tracks for auxiliary motors, and/ or serve as connectors and scaffolds to position and stabilize other materials. -

The First Cells Were Most Likely Very Simple Prokaryotic Forms. Ra- Spirochetes
T HE O RIGIN OF E UKARYOTIC C ELLS The first cells were most likely very simple prokaryotic forms. Ra- spirochetes. Ingestion of prokaryotes that resembled present-day diometric dating indicates that the earth is 4 to 5 billion years old cyanobacteria could have led to the endosymbiotic development of and that prokaryotes may have arisen more than 3.5 billion years chloroplasts in plants. ago. Eukaryotes are thought to have first appeared about 1.5 billion Another hypothesis for the evolution of eukaryotic cells proposes years ago. that the prokaryotic cell membrane invaginated (folded inward) to en- The eukaryotic cell might have evolved when a large anaerobic close copies of its genetic material (figure 1b). This invagination re- (living without oxygen) amoeboid prokaryote ingested small aerobic (liv- sulted in the formation of several double-membrane-bound entities ing with oxygen) bacteria and stabilized them instead of digesting them. (organelles) in a single cell. These entities could then have evolved This idea is known as the endosymbiont hypothesis (figure 1a) and into the eukaryotic mitochondrion, nucleus, and chloroplasts. was first proposed by Lynn Margulis, a biologist at Boston Univer- Although the exact mechanism for the evolution of the eu- sity. (Symbiosis is an intimate association between two organisms karyotic cell will never be known with certainty, the emergence of of different species.) According to this hypothesis, the aerobic bac- the eukaryotic cell led to a dramatic increase in the complexity and teria developed into mitochondria, which are the sites of aerobic diversity of life-forms on the earth. At first, these newly formed eu- respiration and most energy conversion in eukaryotic cells. -

Four-Stranded Mini Microtubules Formed by Prosthecobacter Btubab Show Dynamic Instability
Four-stranded mini microtubules formed by Prosthecobacter BtubAB show dynamic instability Xian Denga,1, Gero Finka,1, Tanmay A. M. Bharata, Shaoda Hea, Danguole Kureisaite-Cizienea, and Jan Löwea,2 aStructural Studies Division, Medical Research Council Laboratory of Molecular Biology, Cambridge CB2 0QH, United Kingdom Edited by Wolfgang Baumeister, Max Planck Institute of Chemistry, Martinsried, Germany, and approved June 1, 2017 (received for review March 27, 2017) Microtubules, the dynamic, yet stiff hollow tubes built from the low resolution currently attainable with the method of electron αβ-tubulin protein heterodimers, are thought to be present only in tomography of entire cells as was used previously (8). eukaryotic cells. Here, we report a 3.6-Å helical reconstruction Approximate helical parameters were deduced by manual in- electron cryomicroscopy structure of four-stranded mini microtu- spection of the subtomogram-averaged map (Fig. 1C) and were bules formed by bacterial tubulin-like Prosthecobacter dejongeii used to obtain a 4.2-Å map of the filaments through iterative BtubAB proteins. Despite their much smaller diameter, mini micro- helical real space reconstruction (13) in RELION (Fig. S1). tubules share many key structural features with eukaryotic micro- Refined helical parameters were: twist −90.7° and rise 10.4 Å. tubules, such as an M-loop, alternating subunits, and a seam that These symmetry operators averaged BtubA and BtubB map re- breaks overall helical symmetry. Using in vitro total internal re- gions and, hence, indicated that the overall structure is not he- flection fluorescence microscopy, we show that bacterial mini lical, as explained in Fig. 1G. Applying the twist and rise four microtubules treadmill and display dynamic instability, another times around the tube means that the nature of the subunits must hallmark of eukaryotic microtubules. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility Thomas Risler1 1Laboratoire Physicochimie Curie (CNRS-UMR 168), Institut Curie, Section de Recherche, 26 rue d’Ulm, 75248 Paris Cedex 05, France (Dated: January 23, 2008) PACS numbers: 1 Article Outline Glossary I. Definition of the Subject and Its Importance II. Introduction III. The Diversity of Cell Motility A. Swimming B. Crawling C. Extensions of cell motility IV. The Cell Cytoskeleton A. Biopolymers B. Molecular motors C. Motor families D. Other cytoskeleton-associated proteins E. Cell anchoring and regulatory pathways F. The prokaryotic cytoskeleton V. Filament-Driven Motility A. Microtubule growth and catastrophes B. Actin gels C. Modeling polymerization forces D. A model system for studying actin-based motility: The bacterium Listeria mono- cytogenes E. Another example of filament-driven amoeboid motility: The nematode sperm cell VI. Motor-Driven Motility A. Generic considerations 2 B. Phenomenological description close to thermodynamic equilibrium C. Hopping and transport models D. The two-state model E. Coupled motors and spontaneous oscillations F. Axonemal beating VII. Putting It Together: Active Polymer Solutions A. Mesoscopic approaches B. Microscopic approaches C. Macroscopic phenomenological approaches: The active gels D. Comparisons of the different approaches to describing active polymer solutions VIII. Extensions and Future Directions Acknowledgments Bibliography Glossary Cell Structural and functional elementary unit of all life forms. The cell is the smallest unit that can be characterized as living. Eukaryotic cell Cell that possesses a nucleus, a small membrane-bounded compartment that contains the genetic material of the cell. Cells that lack a nucleus are called prokaryotic cells or prokaryotes. Domains of life archaea, bacteria and eukarya - or in English eukaryotes, and made of eukaryotic cells - which constitute the three fundamental branches in which all life forms are classified. -

Prokaryotes, Protists, Reconstructing the Photosynthesis, Evolution of Living Things
Prokaryotes, Protists, Reconstructing the Photosynthesis, evolution of living things Endosymbiosis • Systematists study evolutionary relationships Ch 28 & 29 •Look for shared derived (=different from ancestor) traits to group organisms • Evidence used: morphology, 26 February 2009 development, and molecular data (especially DNA sequences) ECOL 182R UofA K. E. Bonine 1 2 Why can’t we figure it out perfectly? • More distant history is obscured by more changes trypanosomes • Among oldest lineages of Bacteria and Archaea in particular, lots of “lateral gene transfer.” Makes it difficult to infer relationships from phylogeny of red blood cells single genes. 3 4 Early prokaryote fossil Diversity of Prokaryotes Bacteria & Archaea 5 6 1 What are microbes? Life can be divided into 3 domains • Only a minority make us sick •Robert Koch, Germ Theory of Disease • In ordinary English, might be anything small 3.8bya –bacteria 1.5bya –yeast –protists – viruses •Prokaryotes = bacteria + archaea • In science, classify by evolutionary • Prokaryote was ancestral and only form for relationships… 7 billions of years 8 Eukarya Scheme has been revised before: Haeckel (1894) Whittaker (1959) Woese (1977) Woese (1990) Three kingdoms Five kingdoms Six kingdoms Three domains Eubacteria Bacteria Monera (prokaryotes) Protista Archaebacteria Archaea Protista Protista Fungi Fungi Plantae Eukarya are Prokaryotes monophyletic, Plantae Plantae Animalia Animalia Animalia paraphyleticparaphyletic, polyphyletic? 9 modified from Wikipedia 10 Shared by all 3 domains Unique to -

Prokaryote Vs. Eukaryote Campaign Biology Name: ______Period: ______
Prokaryote vs. Eukaryote Campaign Biology Name: ___________________________________________ Period: ____________ Prokaryotes vs. Eukaryotes: A Campaign for Election as Coolest Cell Type! Purpose: While prokaryotes and eukaryotes share some similar structures and characteristics of life, the two lead some pretty different lives! Your job in this assignment is to create a campaign poster and a campaign platform commercial for a prokaryote or a eukaryote as they campaign to be elected coolest cell type! Directions: Each group will be assigned a campaign platform from those listed below. Use the resources listed (as well as any others you might find) to understand why your platform makes your cell type the coolest. Create a campaign poster (using one slide on a Google Presentation, shared between partners, and submitted by due date via a form) and a short campaign platform commercial (given as a speech in class) using the attached rubric. Prokaryotic campaign platforms: 1. Ability to form endospores http://goo.gl/Y1tUK http://goo.gl/DhVNe 2. Ability to form biofilms with other cells http://goo.gl/umy7Z http://goo.gl/m9d5W 3. Quorum sensing neighboring cells http://goo.gl/DSiJU http://goo.gl/IFFtA 4. Production of bacteriocins http://goo.gl/lW75y http://goo.gl/WzFv1 5. Endosymbiotic theory http://goo.gl/6VIba http://goo.gl/uSYh4 Eukaryotic campaign platforms: 6. Contain organelles that have their own genetic material in addition to that found in the nucleus http://goo.gl/6VIba http://goo.gl/Nqesq 7. Vesicles http://goo.gl/bwF3x http://goo.gl/j3qY3 8. Membrane bound organelles that facilitate transport (endomembrane system) http://goo.gl/j3qY3 http://goo.gl/i4brZ 9. -

Genscript Product Catalog 2010-2011
GenScript Product Catalog 2010-2011 GenScript Product Catalog GenScript USA Inc. 120 Centennial Ave. Piscataway, NJ 08854 USA Tel: 1-732-885-9188 / 1-732-885-9688 Toll-Free Tel: 1-877-436-7274 Fax: 1-732-210-0262 / 1-732-885-5878 Email: [email protected] Business Development Tel: 1-732-317-5088 Email: [email protected] 2010-2011 GenScript GenScript The Biology CRO The Biology CRO Date Version: 04/21/2010 Your Innovation Partner in Drug Discovery! Welcome to GenScript GenScript USA Incorporation, founded in 2002, is a fast growing biotechnology company and contract research organization (CRO) specialized in custom services and consumable products for academic and pharmaceutical research. Built on our assembly-line mode, one-stop solution, continuous improvement, and stringent IP protection, GenScript provides a comprehensive portfolio of products and services at the most competitive prices in the industry to meet your research needs every day. Over the years, GenScript’s scientists have developed many innovative technologies that allow us to maintain the position at the cutting edge of biological and medical research while offering cost-effective solutions for customers to accelerate their research. Our advanced expertise includes proprietary technology for custom gene synthesis, OptimumGeneTM codon optimization technology, CloneEZ® seamless cloning technology, FlexPeptideTM technology for custom peptide synthesis, BacPowerTM technology for protein expression and purification, T-MaxTM adjuvant and advanced nanotechnology for custom antibody production, as well as ONE-HOUR WesternTM detection system and eStainTM protein staining system. GenScript offers a broad range of reagents, optimized kits, and system solutions to help you unravel the mysteries of biology. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

NIH Public Access Author Manuscript Cell Signal
NIH Public Access Author Manuscript Cell Signal. Author manuscript; available in PMC 2012 December 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Cell Signal. 2011 December ; 23(12): 1907±1920. doi:10.1016/j.cellsig.2011.07.023. Management of cytoskeleton architecture by molecular chaperones and immunophilins Héctor R. Quintáa, Natalia M. Galignianaa, Alejandra G. Erlejmanb, Mariana Lagadaria, Graciela Piwien Pilipuka, and Mario D. Galignianaa,b,* aInstituto de Biología y Medicina Experimental-CONICET, Vuelta de Obligado 2490, Buenos Aires (C1428ADN), Argentina. bDepartamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, Ciudad Universitaria, Universidad de Buenos Aires, Buenos Aires (C1428EGA), Argentina. Abstract Cytoskeletal structure is continually remodeled to accommodate normal cell growth and to respond to pathophysiological cues. As a consequence, several cytoskeleton-interacting proteins become involved in a variety of cellular processes such as cell growth and division, cell movement, vesicle transportation, cellular organelle location and function, localization and distribution of membrane receptors, and cell-cell communication. Molecular chaperones and immunophilins are counted among the most important proteins that interact closely with the cytoskeleton network, in particular with microtubules and microtubule-associated factors. In several situations, heat-shock proteins and immunophilins work together as a functionally active heterocomplex, -

Origin of the Cell Nucleus, Mitosis and Sex: Roles of Intracellular Coevolution Thomas Cavalier-Smith*
Cavalier-Smith Biology Direct 2010, 5:7 http://www.biology-direct.com/content/5/1/7 RESEARCH Open Access Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution Thomas Cavalier-Smith* Abstract Background: The transition from prokaryotes to eukaryotes was the most radical change in cell organisation since life began, with the largest ever burst of gene duplication and novelty. According to the coevolutionary theory of eukaryote origins, the fundamental innovations were the concerted origins of the endomembrane system and cytoskeleton, subsequently recruited to form the cell nucleus and coevolving mitotic apparatus, with numerous genetic eukaryotic novelties inevitable consequences of this compartmentation and novel DNA segregation mechanism. Physical and mutational mechanisms of origin of the nucleus are seldom considered beyond the long- standing assumption that it involved wrapping pre-existing endomembranes around chromatin. Discussions on the origin of sex typically overlook its association with protozoan entry into dormant walled cysts and the likely simultaneous coevolutionary, not sequential, origin of mitosis and meiosis. Results: I elucidate nuclear and mitotic coevolution, explaining the origins of dicer and small centromeric RNAs for positionally controlling centromeric heterochromatin, and how 27 major features of the cell nucleus evolved in four logical stages, making both mechanisms and selective advantages explicit: two initial stages (origin of 30 nm chromatin fibres, enabling DNA compaction; and firmer attachment of endomembranes to heterochromatin) protected DNA and nascent RNA from shearing by novel molecular motors mediating vesicle transport, division, and cytoplasmic motility. Then octagonal nuclear pore complexes (NPCs) arguably evolved from COPII coated vesicle proteins trapped in clumps by Ran GTPase-mediated cisternal fusion that generated the fenestrated nuclear envelope, preventing lethal complete cisternal fusion, and allowing passive protein and RNA exchange. -

Cell Theory VOCABULARY Unit 2: Cells Unit 2: > RE B
3.1 Cell Theory KEY CONCEPT Cells are the basic unit of life. VOCABULARY cell theory MAIN IDEAS cytoplasm Early studies led to the development of the cell theory. organelle Prokaryotic cells lack a nucleus and most internal structures of eukaryotic cells. prokaryotic cell eukaryotic cell Connect to Your World You and all other organisms are made of cells. As you saw on the previous page, > a cell’s structure is closely related to its function. Today we know that cells are the smallest unit of living matter that can carry out all processes required for life. But before the 1600s, people had many other ideas about the basis of life. Like many breakthroughs, the discovery of cells was aided by the development of new technology—in this case, the microscope. MAIN IDEA Early studies led to the development of the READING TOOLBOX cell theory. TAKING NOTES As you read, make an outline Almost all cells are too small to see without the aid of a microscope. Although using the headings as topics. glass lenses had been used to magnify images for hundreds of years, the early Summarize details that further lenses were not powerful enough to reveal individual cells. The invention of explain those ideas. the compound microscope in the late 1500s was an early step toward this I. Main Idea discovery. The Dutch eyeglass maker Zacharias Janssen, who was probably A. Supporting idea assisted by his father, Hans, usually gets credit for this invention. 1. Detail A compound microscope contains two or more lenses. Total magnification, the 2.