The Future of Endangered Crayfish in Light of Protected Areas and Habitat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Status of the Endangered Freshwater Fishes in China and the Analysis of the Endangered Causes Institute of Hydrobiology
The status of the endangered freshwater fishes in China and The analysis of the endangered causes HE Shunping, CIIEN Yiyu Institute of Hydrobiology, CAS, Wuhan, ITubei Province, 430072 Abstract More than 800 species of freshwater fishes are precious biological resources in inland water system of China. Among them, there are a great number of endemic and precious group, and a lot of monotypic genera and species. Recently, owing to the synthetic effects of the natural and human-beings, many of these fishes gradually became endangered. The preliminary statistic result indicates that 92 species are endangered fishes and account for 10% of the total freshwater fishes in China. For the purpose of protection of the biodiversity of fishes, it is necessary to analyse these causes which have led the fishes to become endangered. This report could be used as a scientific reference for researching and saving the endemic precious freshwater fishes in China. Key words Endangered freshwater fishes, Endangered causes, China In the process of the evolution of living things, along with the origin of life, the extinction of life also existed. In the long_ life history, the speciation and the extinction of living things often keep a relative balance. As time goes on, especially after by the impact of human beings activity of production and life, the pattern of the biodiversity were changed or damaged, more or less. At last, in the modern society, human beings activity not only accelerate the progress of society and the development of economy, but also, as a special species, become the source of disturbing_ to other species. -

Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic
water Review Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic Valentina S. Artamonova 1, Ivan N. Bolotov 2,3,4, Maxim V. Vinarski 4 and Alexander A. Makhrov 1,4,* 1 A. N. Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia; [email protected] 2 Laboratory of Molecular Ecology and Phylogenetics, Northern Arctic Federal University, 163002 Arkhangelsk, Russia; [email protected] 3 Federal Center for Integrated Arctic Research, Russian Academy of Sciences, 163000 Arkhangelsk, Russia 4 Laboratory of Macroecology & Biogeography of Invertebrates, Saint Petersburg State University, 199034 Saint Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Analysis of zoogeographic, paleogeographic, and molecular data has shown that the ancestors of many fresh- and brackish-water cold-tolerant hydrobionts of the Mediterranean region and the Danube River basin likely originated in East Asia or Central Asia. The fish genera Gasterosteus, Hucho, Oxynoemacheilus, Salmo, and Schizothorax are examples of these groups among vertebrates, and the genera Magnibursatus (Trematoda), Margaritifera, Potomida, Microcondylaea, Leguminaia, Unio (Mollusca), and Phagocata (Planaria), among invertebrates. There is reason to believe that their ancestors spread to Europe through the Paratethys (or the proto-Paratethys basin that preceded it), where intense speciation took place and new genera of aquatic organisms arose. Some of the forms that originated in the Paratethys colonized the Mediterranean, and overwhelming data indicate that Citation: Artamonova, V.S.; Bolotov, representatives of the genera Salmo, Caspiomyzon, and Ecrobia migrated during the Miocene from I.N.; Vinarski, M.V.; Makhrov, A.A. -

Study of Heavy Metals Existing in the Danube Wa- Ters in Turnu Severin – Bechet Section
South Western Journal of Vol.2, No.1, 2011 Horticulture, Biology and Environment pp.47-55 P-Issn: 2067- 9874, E-Issn: 2068-7958 STUDY OF HEAVY METALS EXISTING IN THE DANUBE WA- TERS IN TURNU SEVERIN – BECHET SECTION Elena GAVRILESCU University of Craiova, Horticulture Faculty, A.I.Cuza Street, no. 13, Craiova, Romania E-mail: [email protected] Abstract. The Danube River Protection Convention and the Environment Programme of the Danube River Basin aim to the complex assessment of water quality at the national level and of its development trends in order to substantiate the measures and policies to reduce pollution, plus other pri- ority objectives as the quantification of the heavy metals content (Lack 1997). The monitored sections in the study, respectively Turnu Severin - Calafat - Bechet were part of the TNMN network for tracking the Danube water quality (Harmancioglu et al. 1997).The heavy metals are from both the upstream of Turnu Severin and from the Jiu River. After the study conducted in 2007-2009 there were found in some metals significant amounts of nickel, copper, chromium, arsenic and lead in particular. Key words: monitoring, heavy metals, aquatic ecosystem, water pollution, the Danube INTRODUCTION Danube represents the biggest water resource for Romania being more than double (85x109 cm/year) in comparison with the inland water (river and lakes), which represents about 40x109 cm/year, but the possibilities of their use in natural regime are limited because of different technical reasons (Botterweg & Rodda 1999). Even so the importance of the Danube health is of major concern for Romania as well as for other countries. -

Evolutionary Genomics of a Plastic Life History Trait: Galaxias Maculatus Amphidromous and Resident Populations
EVOLUTIONARY GENOMICS OF A PLASTIC LIFE HISTORY TRAIT: GALAXIAS MACULATUS AMPHIDROMOUS AND RESIDENT POPULATIONS by María Lisette Delgado Aquije Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia August 2021 Dalhousie University is located in Mi'kma'ki, the ancestral and unceded territory of the Mi'kmaq. We are all Treaty people. © Copyright by María Lisette Delgado Aquije, 2021 I dedicate this work to my parents, María and José, my brothers JR and Eduardo for their unconditional love and support and for always encouraging me to pursue my dreams, and to my grandparents Victoria, Estela, Jesús, and Pepe whose example of perseverance and hard work allowed me to reach this point. ii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................ vii LIST OF FIGURES ........................................................................................................... ix ABSTRACT ...................................................................................................................... xii LIST OF ABBREVIATION USED ................................................................................ xiii ACKNOWLEDGMENTS ................................................................................................ xv CHAPTER 1. INTRODUCTION ....................................................................................... 1 1.1 Galaxias maculatus .................................................................................................. -

Revalidation and Redescription of Brachymystax Tsinlingensis Li, 1966 (Salmoniformes: Salmonidae) from China
Zootaxa 3962 (1): 191–205 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3962.1.12 http://zoobank.org/urn:lsid:zoobank.org:pub:F7864FFE-F182-455E-B37A-8A253D8DB72D Revalidation and redescription of Brachymystax tsinlingensis Li, 1966 (Salmoniformes: Salmonidae) from China YING-CHUN XING1,2, BIN-BIN LV3, EN-QI YE2, EN-YUAN FAN1, SHI-YANG LI4, LI-XIN WANG4, CHUN- GUANG ZHANG2,* & YA-HUI ZHAO2,* 1Natural Resource and Environment Research Center, Chinese Academy of Fishery Sciences, Beijing, China. 2Institute of Zoology, Chinese Academy of Sciences, Beijing, China. 3Yellow River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Xi’an, China. 4The College of Forestry of Beijing Forestry University, Beijing, China. *Corresponding authors: Yahui Zhao, [email protected]; Chunguang Zhang, [email protected] Abstract Brachymystax tsinlingensis Li, 1966 is revalidated and redescribed. It can be distinguished from all congeners by the fol- lowing combination of characteristics: no spots on operculum; gill rakers 15-20; lateral-line scales 98-116; pyloric caeca 60-71. Unique morphological characters and genetic divergence of this species are discussed. This species has a limited distribution in several streams of the middle part of the Qinling Mountains in China. Methods for management and pro- tection of B. tsinlingensis need to be re-evaluated. Key words: Brachymystax, revalidation, redescription, Salmonidae, China Introduction The genus Brachymystax Günther, 1866, belonging to Salmonidae, Salmoniformes, is distributed in eastern and northern Asia with three currently recognized valid species (Froese & Pauly, 2014): B. -

Long-Term Trends in Water Quality Indices in the Lower Danube and Tributaries in Romania (1996–2017)
International Journal of Environmental Research and Public Health Article Long-Term Trends in Water Quality Indices in the Lower Danube and Tributaries in Romania (1996–2017) Rodica-Mihaela Frîncu 1,2 1 National Institute for Research and Development in Chemistry and Petrochemistry—ICECHIM, 202 Splaiul Independentei, 060021 Bucharest, Romania; [email protected]; Tel.: +40-21-315-3299 2 INCDCP ICECHIM Calarasi Branch, 2A Ion Luca Caragiale St., 910060 Calarasi, Romania Abstract: The Danube River is the second longest in Europe and its water quality is important for the communities relying on it, but also for supporting biodiversity in the Danube Delta Biosphere Reserve, a site with high ecological value. This paper presents a methodology for assessing water quality and long-term trends based on water quality indices (WQI), calculated using the weighted arithmetic method, for 15 monitoring stations in the Lower Danube and Danube tributaries in Romania, based on annual means of 10 parameters for the period 1996–2017. A trend analysis is carried out to see how WQIs evolved during the studied period at each station. Principal component analysis (PCA) is applied on sub-indices to highlight which parameters have the highest contributions to WQI values, and to identify correlations between parameters. Factor analysis is used to highlight differences between locations. The results show that water quality has improved significantly at most stations during the studied period, but pollution is higher in some Romanian tributaries than in the Danube. The parameters with the highest contribution to WQI are ammonium and total phosphorus, suggesting the need to continue improving wastewater treatment in the studied area. -

Settlement History and Sustainability in the Carpathians in the Eighteenth and Nineteenth Centuries
Munich Personal RePEc Archive Settlement history and sustainability in the Carpathians in the eighteenth and nineteenth centuries Turnock, David Geography Department, The University, Leicester 21 June 2005 Online at https://mpra.ub.uni-muenchen.de/26955/ MPRA Paper No. 26955, posted 24 Nov 2010 20:24 UTC Review of Historical Geography and Toponomastics, vol. I, no.1, 2006, pp 31-60 SETTLEMENT HISTORY AND SUSTAINABILITY IN THE CARPATHIANS IN THE EIGHTEENTH AND NINETEENTH CENTURIES David TURNOCK* ∗ Geography Department, The University Leicester LE1 7RH, U.K. Abstract: As part of a historical study of the Carpathian ecoregion, to identify salient features of the changing human geography, this paper deals with the 18th and 19th centuries when there was a large measure political unity arising from the expansion of the Habsburg Empire. In addition to a growth of population, economic expansion - particularly in the railway age - greatly increased pressure on resources: evident through peasant colonisation of high mountain surfaces (as in the Apuseni Mountains) as well as industrial growth most evident in a number of metallurgical centres and the logging activity following the railway alignments through spruce-fir forests. Spa tourism is examined and particular reference is made to the pastoral economy of the Sibiu area nourished by long-wave transhumance until more stringent frontier controls gave rise to a measure of diversification and resettlement. It is evident that ecological risk increased, with some awareness of the need for conservation, although substantial innovations did not occur until after the First World War Rezumat: Ca parte componentă a unui studiu asupra ecoregiunii carpatice, pentru a identifica unele caracteristici privitoare la transformările din domeniul geografiei umane, acest articol se referă la secolele XVIII şi XIX când au existat măsuri politice unitare ale unui Imperiu Habsburgic aflat în expansiune. -

Introduceţi Titlul Lucrării
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Annals of the University of Craiova - Agriculture, Montanology, Cadastre Series Analele Universităţii din Craiova, seria Agricultură – Montanologie – Cadastru (Annals of the University of Craiova - Agriculture, Montanology, Cadastre Series) Vol. XLIII 2013 RESEARCH ON THE IDENTIFICATION AND PROMOTION OF AGROTURISTIC POTENTIAL OF TERRITORY BETWEEN JIU AND OLT RIVER CĂLINA AUREL, CĂLINA JENICA, CROITORU CONSTANTIN ALIN University of Craiova, Faculty of Agriculture and Horticulture Keywords: agrotourism, agrotourism potential, agrotouristic services, rural area. ABSTRACT The idea of undertaking this research emerged in 1993, when was taking in study for doctoral thesis region between Jiu and Olt River. Starting this year, for over 20 years, I studied very thoroughly this area and concluded that it has a rich and diverse natural and anthropic tourism potential that is not exploited to its true value. Also scientific researches have shown that the area benefits of an environment with particular beauty and purity, of an ethnographic and folklore thesaurus of great originality and attractiveness represented by: specific architecture, traditional crafts, folk techniques, ancestral habits, religion, holidays, filled with historical and art monuments, archeological sites, museums etc.. All these natural and human tourism resources constitute a very favorable and stimulating factor in the implementation and sustained development of agritourism and rural tourism activities in the great and the unique land between Jiu and Olt River. INTRODUCTION Agritourism and rural tourism as economic and socio-cultural activities are part of protection rules for built and natural environment, namely tourism based on ecological principles, became parts of ecotourism, which as definition and content goes beyond protected areas (Grolleau H., 1988 and Annick Deshons, 2006). -

Distribution and Heterogeneity of Heterochromatin in the European Huchen
PL-ISSN0015-5497(print),ISSN1734-9168(online) FoliaBiologica(Kraków),vol.62(2014),No2 Ó InstituteofSystematicsandEvolutionofAnimals,PAS,Kraków, 2014 doi:10.3409/fb62_2.81 DistributionandHeterogeneityofHeterochromatinintheEuropean Huchen(Huchohucho Linnaeus,1758)(Salmonidae)* KUCINSKI Marcin,OCALEWICZ Konrad,FOPP-BAYAT Dorota,LISZEWSKI Tomasz, FURGALA-SELEZNIOW Grazyna,JANKUN Malgorzata AcceptedFebruary19,2014 KUCINSKI M., OCALEWICZ K., FOPP-BAYAT D., LISZEWSKI T., FURGALA-SELEZNIOW G., JANKUN M. 2014. Distribution and heterogeneity of heterochromatinintheEuropeanhuchen (Hucho hucho Linnaeus, 1758) (Salmonidae). Folia Biologica (Kraków) 62: 81-89. The chromosomal characteristics, locations and variations of the heterochromatin were studied in the European huchen (Hucho hucho, Linnaeus, 1758) karyotype using conventional C- banding, endonuclease digestion banding, silver nitrate (AgNO3), chromomycin A3 (CMA3) and DAPIstainingtechniques.Thekaryotypeconsistsof82 chromosomes: 13 pairs of metacentric chromosomes, 2 pairs of submetacentric chromosomes and 26 pairs of subtelo-acrocentric chromosomes (NF=112). Original data on the chromosomal distribution of segments resistant to Alu I, Dde I and Mbo I restriction endonucleases and identification of the C-banded heterochromatin presented here have been used to characterize the huchen karyotype. On the basis of the banding patterns provided in the course of restriction enzyme digestion, AgNO3/CMA3 staining and C-banding we distinguished twelve types of heterochromatin grouped in four areas of the -

Preparing the Shaanxi-Qinling Mountains Integrated Ecosystem Management Project (Cofinanced by the Global Environment Facility)
Technical Assistance Consultant’s Report Project Number: 39321 June 2008 PRC: Preparing the Shaanxi-Qinling Mountains Integrated Ecosystem Management Project (Cofinanced by the Global Environment Facility) Prepared by: ANZDEC Limited Australia For Shaanxi Province Development and Reform Commission This consultant’s report does not necessarily reflect the views of ADB or the Government concerned, and ADB and the Government cannot be held liable for its contents. (For project preparatory technical assistance: All the views expressed herein may not be incorporated into the proposed project’s design. FINAL REPORT SHAANXI QINLING BIODIVERSITY CONSERVATION AND DEMONSTRATION PROJECT PREPARED FOR Shaanxi Provincial Government And the Asian Development Bank ANZDEC LIMITED September 2007 CURRENCY EQUIVALENTS (as at 1 June 2007) Currency Unit – Chinese Yuan {CNY}1.00 = US $0.1308 $1.00 = CNY 7.64 ABBREVIATIONS ADB – Asian Development Bank BAP – Biodiversity Action Plan (of the PRC Government) CAS – Chinese Academy of Sciences CASS – Chinese Academy of Social Sciences CBD – Convention on Biological Diversity CBRC – China Bank Regulatory Commission CDA - Conservation Demonstration Area CNY – Chinese Yuan CO – company CPF – country programming framework CTF – Conservation Trust Fund EA – Executing Agency EFCAs – Ecosystem Function Conservation Areas EIRR – economic internal rate of return EPB – Environmental Protection Bureau EU – European Union FIRR – financial internal rate of return FDI – Foreign Direct Investment FYP – Five-Year Plan FS – Feasibility -

The Danube River Basin District
/ / / / a n ï a r k U / /// ija ven Slo /// o / sk n e v o l S / / / / a r o G a n r C i a j i b r S / / / / a i n â m o R / / / / a v o d l o M / / / / g á z s r ro ya ag M The /// a / blik repu Danube River Ceská / Hrvatska //// osna i Hercegovina //// Ba˘lgarija /// / B /// Basin District h ic e River basin characteristics, impact of human activities and economic analysis required under Article 5, Annex II randr Annex III, and inventory of protected areas required under Article 6, Annex IV of the EU Water Framework Directivee (2000/60/EC) t s Part A – Basin-wide overviewÖ / / Short: “Danube Basin Analysis (WFD Roof Report 2004)” / / d n a l h c s t u e D / / / / The complete report consists of Part A: Basin-wide overview, and Part B: Detailed analysis of the Danube river basin countries 18 March 2005, Reporting deadline: 22 March 2005 Prepared by International Commission for the Protection of the Danube River (ICPDR) in cooperation with the countries of the Danube River Basin District. The Contracting Parties to the Danube River Protection Convention endorsed this report at the 7th Ordinary Meeting of the ICPDR on December 13-14, 2004. The final version of the report was approved 18 March 2005. Overall coordination and editing by Dr. Ursula Schmedtje, Technical Expert for River Basin Management at the ICPDR Secretariat, under the guidance of the River Basin Management Expert Group. ICPDR Document IC/084, 18 March 2005 International Commission for the Protection of the Danube River Vienna International Centre D0412 P.O. -
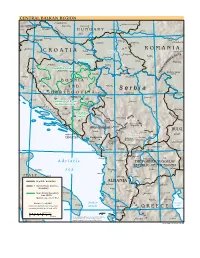
S E R B I a Knin ˆ Bor
CENTRAL BALKAN REGION 16 18 20 22 Nagykanizsa Tisza Hódmezövásárhely Dravaˆ Kaposvár Szekszárd SLOVENIA P Celje Varazdin A Szeged N H U N G A R Y N Arad O N Pécs 46 I 46 A Danube Subotica Mures N Bjelovar B A Zagreb S Kikinda Deva I Tisa N Sombor Timisoara¸ Hunedoara T N A Karlovac B A R O M A N I A Sisak C R O A T I A Osijek Vojvodina Petroseni Sava Vukovar Zrenjanin S Resita¸ ¸ LP Novi Sad A ˆ N IA Slavonski Brod Federation of Bosnia Vrsac N and Herzegovina Danube A Tirgu-Jiu V Prijedor Ruma L ˆ ˆ ˆ Y S Bihac Republika Srpska Brcko Pancevo N A D Banja Luka Doboj Sava R Drobeta-Turnu Bijeljina Sabac Belgrade Danube T Severin Udbina I Smederevo Kljuc Tuzla N B O S N I A A A N D Valjevo Danube Zenica Drina R S e r b i a Knin ˆ Bor 44 H E R Z E G O V I N A Srebrenica Kragujevac 44 Glamoc I ˆ Vidin Calafat C Sarajevo Uzice Paracin´ Šibenik Pale Kraljevo Federation of Bosnia ˆ Morava D and Herzegovina Gorazde Split A ˆ L A M Foca Montana A T L Nis´ B I Republika A Mostar L A Priboj K P Srpska A ˆ Ta ra Novi Pazar N M Ploce S Bijelo TS. Piva Polje Neum Kosovska Mitrovica Berane Montenegro BULG. Nikšic´ Pec´ Priština Dubrovnik Kosovo Vranje Pernik CROATIA Podgorica Dakovica Gnjilane NORTH (Djakovica) Uroševac Kotor ALBANIAN Kyustendil ALPS Prizren A Lake I N Kumanovo Scutari N Kukës A 42 Shkodër L Tetovo Skopje 42 Bar P R A S Gostivar Štip Shëngjin Titov Veles A d r i a t i c Peshkopi THE FORMER YUGOSLAV REPUBLIC OF MACEDONIA Vardar Strumica Barletta S e a Tirana Prilep Lake Durrës Ohrid I T A L Y Bari Elbasan Ohrid Bitola Republic boundary