Glyphosate-Monograph.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Occurrence of Shell Disease and Carapace Abnormalities on Natural
JMBA2 - Biodiversity Records Published on-line Occurrence of shell disease and carapace abnormalities on natural population of Neohelice granulata (Crustacea: Varunidae) from a tropical mangrove forest, Brazil Rafael Augusto Gregati* and Maria Lucia Negreiros Fransozo NEBECC (Group of Studies on Crustacean Biology, Ecology and Culture), Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista (UNESP) 18618-000, Botucatu, SP, Brazil. *Corresponding author, e-mail: [email protected] In this note, we registered the occurrence of shell diseases and carapace abnormalities on a natural population of Neohelice granulata from a tropical mangrove forest, in South America, as a part of a wide ecological study. The occurrence of 32 adult crabs (1.77%) with black or brown spotted shell, or deformities on carapace was registered, collected in the autumn and winter seasons. The low prevalence of shell diseases and abnormalities in this natural population is considered normal and probably caused by injuries occurred during the moult period of crabs. Keywords: Neohelice granulata, shell disease, carapace abnormalities Introduction Shell diseases and external abnormalities or deformities are just one of the common problems affecting freshwater and marine crustaceans. These diseases have been reported in many natural crustacean populations, principally in many species of economic importance such as the Alaskan king crab, portunid crabs, shrimps and lobsters (Maloy, 1978; Sindermann, 1989; Noga et al., 2000). The shell diseases are characterized by various types of erosive lesions on the carapace (Johnson, 1983; Sindermann & Lightner, 1988) and the classical and most common kind of shell disease is know as ‘brown spot’ or ‘black spot’, which consists of various-sized foci of hyperpigmentation (Rosen, 1970; Noga et al., 2000). -

Natural Diet of Neohelice Granulata (Dana, 1851) (Crustacea, Varunidae) in Two Salt Marshes of the Estuarine Region of the Lagoa Dos Patos Lagoon
91 Vol.54, n. 1: pp. 91-98, January-February 2011 BRAZILIAN ARCHIVES OF ISSN 1516-8913 Printed in Brazil BIOLOGY AND TECHNOLOGY AN INTERNATIONAL JOURNAL Natural Diet of Neohelice granulata (Dana, 1851) (Crustacea, Varunidae) in Two Salt Marshes of the Estuarine Region of the Lagoa dos Patos Lagoon Roberta Araujo Barutot 1*, Fernando D´Incao 1 and Duane Barros Fonseca 2 1Instituto de Oceanografia; Universidade Federal do Rio Grande; 96201-900; Rio Grande - RS - Brasil. 2Instituto de Ciências Biológicas; Universidade Federal do Rio Grande; 96201-900; Rio Grande - RS - Brasil ABSTRACT Natural diet of Neohelice granulata in two salt marshes of Lagoa dos Patos, RS were studied. Sampling was performed seasonally and crabs were captured by hand by three persons during one hour, fixed in formaldehyde (4%) during 24 h, transferred to alcohol (70%). Each foregut was weighed and repletion level was determined. Differences between sexes in the frequencies of occurrence of items were tested by χ2test. A total of 452 guts were analyzed. Quali-quantitative analyses were calculated following the method of relative frequency occurrence and relative frequency of the points. At both sites, for both sexes and in all seasons, the main food items were sediment, Spartina sp. and plant detritus. The highest values of mean repletion index were estimated for the spring and summer. Analysing both salt marshes, in different seasons significant shifts in the natural diet of Neohelice granulata was not observed throughout the period of study. Key words : Crustacea, Brachyura, diet, salt marsh INTRODUCTION Neohelice granulata (Dana, 1851) is a crab found in salt marshes and mangroves of the Southern Clarification of trophic relationships is one Atlantic Coast, from Rio de Janeiro (Brazil) to important approach for understanding the Patagonia (Argentina) (Melo, 1996), and it is one organization of communities. -

Growth, Tolerance to Low Salinity, and Osmoregulation in Decapod Crustacean Larvae
Vol. 12: 249–260, 2011 AQUATIC BIOLOGY Published online June 1 doi: 10.3354/ab00341 Aquat Biol Growth, tolerance to low salinity, and osmoregulation in decapod crustacean larvae Gabriela Torres1, 2,*, Luis Giménez1, Klaus Anger2 1School of Ocean Sciences, Bangor University, Menai Bridge, LL59 5AB, UK 2Biologische Anstalt Helgoland, Foundation Alfred Wegener Institute for Polar and Marine Research, 27498 Helgoland, Germany ABSTRACT: Marine invertebrate larvae suffer high mortality due to abiotic and biotic stress. In planktotrophic larvae, mortality may be minimised if growth rates are maximised. In estuaries and coastal habitats however, larval growth may be limited by salinity stress, which is a key factor select- ing for particular physiological adaptations such as osmoregulation. These mechanisms may be ener- getically costly, leading to reductions in growth. Alternatively, the metabolic costs of osmoregulation may be offset by the capacity maintaining high growth at low salinities. Here we attempted identify general response patterns in larval growth at reduced salinities by comparing 12 species of decapod crustaceans with differing levels of tolerance to low salinity and differing osmoregulatory capability, from osmoconformers to strong osmoregulators. Larvae possessing tolerance to a wider range in salinity were only weakly affected by low salinity levels. Larvae with a narrower tolerance range, by contrast, generally showed reductions in growth at low salinity. The negative effect of low salinity on growth decreased with increasing osmoregulatory capacity. Therefore, the ability to osmoregulate allows for stable growth. In euryhaline larval decapods, the capacity to maintain high growth rates in physically variable environments such as estuaries appears thus to be largely unaffected by the energetic costs of osmoregulation. -

Pesticide Action Networknews
30th Anniversary Edition Pesticide Action Network NEWS Advancing alternatives to pesticides worldwide • www.panna.org Year-end 2012 Cultivating the roots of health and justice Pesticide Action Network: The First 30 Years By 1982, the luster of industrial agriculture—the so-called “Green Revolution”—had faded in developing countries. The promised dramatic increases in yields from “miracle” hybrid grains that required high inputs of water, chemical fertilizers and pesticides failed to deliver and were revealed as campaigns to sell technology to people who couldn’t afford it. Local communities were losing control over their own food systems, and women and children shouldered more of the fieldwork—and bore the brunt of pesticide exposure. The global pesticide trade was, however, yielding dramatic profits for chemical companies as more and more farmers were trapped on a pesticide treadmill. That was the world when PAN was founded. In the years since, the world community has reassessed. When rice farming was collapsing in the 1980s due to pest resurgence from resistance to pesticides, Indonesia needed alternatives. A com- ifty years after Silent Spring and 30 years bination of community-scale peer-learning projects recaptured Fafter PAN’s founding, our struggle for health Indigenous farming knowledge and wove it into new ecological pest management. “Farmer Field Schools”—today adapted to and justice remains vital and more urgent than local needs in many countries—returned bountiful crops of rice ever. Challenging the global proliferation of while expenditures on agrichemicals were slashed. By 2002, more pesticides is about challenging corporate control, than a million Indonesian farmers had participated in field schools that became models for localized sustainable agriculture in other ensuring scientific integrity and defending basic countries. -

Complaint for Declaratory and Injunctive Relief 1 1 2 3 4 5 6 7 8 9
1 Justin Augustine (CA Bar No. 235561) Jaclyn Lopez (CA Bar No. 258589) 2 Center for Biological Diversity 351 California Street, Suite 600 3 San Francisco, CA 94104 Tel: (415) 436-9682 4 Fax: (415) 436-9683 [email protected] 5 [email protected] 6 Collette L. Adkins Giese (MN Bar No. 035059X)* Center for Biological Diversity 8640 Coral Sea Street Northeast 7 Minneapolis, MN 55449-5600 Tel: (651) 955-3821 8 Fax: (415) 436-9683 [email protected] 9 Michael W. Graf (CA Bar No. 136172) 10 Law Offices 227 Behrens Street 11 El Cerrito, CA 94530 Tel: (510) 525-7222 12 Fax: (510) 525-1208 [email protected] 13 Attorneys for Plaintiffs Center for Biological Diversity and 14 Pesticide Action Network North America *Seeking admission pro hac vice 15 16 IN THE UNITED STATES DISTRICT COURT 17 FOR THE NORTHERN DISTRICT OF CALIFORNIA 18 SAN FRANCISCO DIVISION 19 20 CENTER FOR BIOLOGICAL ) 21 DIVERSITY, a non-profit organization; and ) Case No.__________________ PESTICIDE ACTION NETWORK ) 22 NORTH AMERICA, a non-profit ) organization; ) 23 ) Plaintiffs, ) COMPLAINT FOR DECLARATORY 24 ) AND INJUNCTIVE RELIEF v. ) 25 ) ENVIRONMENTAL PROTECTION ) 26 AGENCY; and LISA JACKSON, ) Administrator, U.S. EPA; ) 27 ) Defendants. ) 28 _____________________________________ ) Complaint for Declaratory and Injunctive Relief 1 1 INTRODUCTION 2 1. This action challenges the failure of Defendants Environmental Protection Agency and 3 Lisa Jackson, Environmental Protection Agency Administrator, (collectively “EPA”) to consult with the 4 United States Fish and Wildlife Service (“FWS”) and National Marine Fisheries Service (“NMFS”) 5 (collectively “Service”) pursuant to Section 7(a)(2) of the Endangered Species Act (“ESA”), 16 U.S.C. -

Sound Management of Pesticides and Diagnosis and Treatment Of
* Revision of the“IPCS - Multilevel Course on the Safe Use of Pesticides and on the Diagnosis and Treatment of Presticide Poisoning, 1994” © World Health Organization 2006 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. CONTENTS Preface Acknowledgement Part I. Overview 1. Introduction 1.1 Background 1.2 Objectives 2. Overview of the resource tool 2.1 Moduledescription 2.2 Training levels 2.3 Visual aids 2.4 Informationsources 3. Using the resource tool 3.1 Introduction 3.2 Training trainers 3.2.1 Organizational aspects 3.2.2 Coordinator’s preparation 3.2.3 Selection of participants 3.2.4 Before training trainers 3.2.5 Specimen module 3.3 Trainers 3.3.1 Trainer preparation 3.3.2 Selection of participants 3.3.3 Organizational aspects 3.3.4 Before a course 4. -

Burrowing Activity of the Neohelice Granulata Crab (Brachyura, Varunidae) in Southwest Atlantic Intertidal Areas
Ciencias Marinas (2018), 44(3): 155–167 http://dx.doi.org/10.7773/cm.v44i3.2851 Burrowing activity of the Neohelice granulata crab (Brachyura, Varunidae) in southwest Atlantic intertidal areas Actividad cavadora del cangrejo Neohelice granulata (Brachyura, Varunidae) en sitios intermareales del atlántico sudoccidental Sabrina Angeletti1*, Patricia M Cervellini1, Leticia Lescano2,3 1 Instituto de Ciencias Biológicas y Biomédicas del Sur, Consejo Nacional de Investigaciones Científicas y Técnicas-Universidad Nacional del Sur (CONICET-UNS), San Juan 670, 8000-Bahía Blanca, Argentina. 2 Departamento de Geología, Universidad Nacional del Sur, San Juan 670, 8000-Bahía Blanca, Argentina. 3 Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, Calle 526 entre 10 y 11, 1900-La Plata, Argentina. * Corresponding author. E-mail: [email protected] A. The burrowing and semiterrestrial crab Neohelice granulata actively and constantly builds its burrows in the intertidal zone of the Bahía Blanca Estuary during low tide. Differences in structural morphology of N. granulata burrows and burrowing activities in contrasting microhabitats (saltmarsh and mudflat) were analyzed and related to several conditions, such as tide level, substrate type, sediment properties, and population density. In the mudflat the higher density of total burrows in autumn (172 burrows·m–2) was associated with molt timing, and the higher density of active burrows in summer (144 burrows·m–2) was associated with reproductive migration. Sediments from biogenic mounds (removed by crabs) showed higher water content and penetrability than surface sediments (control), suggesting that bioturbation increases the values of these parameters. Grain size distribution profiles and mineralogical composition did not vary between microhabitats or between seasons. -

Determination of Selected Priority Pesticides in High Water Fruits and Vegetables by Modified Quechers and GC-ECD with GC-MS/MS Confirmation
molecules Article Determination of Selected Priority Pesticides in High Water Fruits and Vegetables by Modified QuEChERS and GC-ECD with GC-MS/MS Confirmation Maciej Tankiewicz Department of Environmental Toxicology, Faculty of Health Sciences with Subfaculty of Nursing and Institute of Maritime and Tropical Medicine, Medical University of Gda´nsk,D˛ebowaStr. 23A, 80-204 Gda´nsk,Poland; [email protected]; Tel.: +48-58-349-1937 Received: 21 December 2018; Accepted: 23 January 2019; Published: 24 January 2019 Abstract: A modified quick, easy, cheap, efficient, rugged and safe (QuEChERS) method coupled to gas chromatography with electron capture detector (GC-ECD) was developed for simultaneous determination of selected electronegative pesticides in fruits and vegetables with high water content. The chosen compounds are commonly detected in fruit and vegetable crops, and some of their metabolites have even been found in human urine. In addition, some of them are known or suspected carcinogens according to the International Agency for Research of Cancer. Extraction and clean up parameters were optimized, thus the original QuEChERS method was modified to decrease solvent usage, in accordance with ‘green chemistry’ principles. The proposed methodology was validated in terms of selectivity, specificity, linearity, precision and accuracy. The obtained limits of detection (LODs) for all investigated pesticides ranged from 5.6 µg·kg−1 to 15 µg·kg−1 and limits of quantification (LOQs) from 17 µg·kg−1 to 45 µg·kg−1. The obtained data demonstrated the good reproducibility and stability of the procedure in the tested concentration range up to 10 mg·kg−1, with relative standard deviations (RSDs) lower than 10%. -

4C Pesticide Lists
4C PESTICIDE LISTS Version 4.0 II 4C PESTICIDE LISTS Copyright notice © 2020 4C Services GmbH This document is protected by copyright. It is freely available from the 4C website or upon request. No part of this copyrighted document may be changed or amended. The document may not be duplicated or copied in any form or by any means for commercial purpose without permission of 4C Services. Document Title: 4C Pesticide Lists Version 4.0 Valid from: 01 July 2020 III Content List of Tables ........................................................................................................................ IV Abbreviations ....................................................................................................................... IV 4C PESTICIDE LISTS 1 Introduction ................................................................................................................... 5 2 Selection Criteria Used for the 4C Pesticide Lists .......................................................... 5 3 4C Red List Pesticides: 4C Code of Conduct Requirements and Actions to be Promoted .................................. 6 4 4C Yellow List Pesticides: 4C Code of Conduct Requirements and Actions to be Promoted .................................. 7 © 4C Services GmbH IV List of Tables Table 1: 4C list of unacceptable pesticides ............................................................................ 8 Table 2: 4C red pesticide list ................................................................................................. 9 Table -
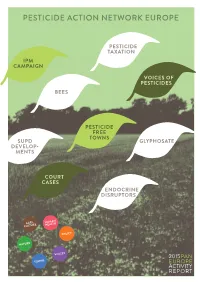
Pesticide Action Network Europe
PESTICIDE ACTION NETWORK EUROPE PESTICIDE TAXATION IPM CAMPAIGN VOICES OF PESTICIDES BEES PESTICIDE FREE TOWNS SUPD GLYPHOSATE DEVELOP- MENTS COURT CASES ENDOCRINE DISRUPTORS AGRI- HUMAN CULTURE HEALTH POLICY NATURE VOICES 2015PAN TOWNS EUROPE ACTIVITY REPORT WHO WE ARE 3 WHAT WE DO 5 PAN EUROPE & THE SUSTAINABLE USE DIRECTIVE 9 INTEGRATED PEST MANAGEMENT 11 PESTICIDE FREE TOWNs 13 BEEs 15 ENDOCRINE DISRUPTING CHEMICALs 17 FURTHER THREATS (GLYPHOSATE, ETC.) 19 COURT CASEs 21 VAT & PESTICIDE TAXATION 23 PESTICIDE SALE 2011-2013 – BASELINE YEAR 2011=100 TONS OF PESTICIDES SOLD IN 2011–2013 WHO WE ARE Pesticide Action Network (PAN) was founded in 1982 and is a network of over 600 non- governmental organisations, institutions and individuals in over 60 countries worldwide working to minimise the negative effects and replace the use of harmful pesticides with eco- logically sound alternatives. Its projects and campaigns are coordinated by five autonomous Regional Centres. PAN Europe is the regional centre in Europe. Located in Brussels, it was founded in 1987 and brings together 34 consumer, public health, and environmental organisations, trades unions, women’s groups and farmer associations from across 21 European countries. PAN Europe’s vision is of a world in which high agricultural productivity is achieved by truly sustainable agricultural production systems in which agrochemical inputs and environmental damage are minimised, and where local people control local production using local varieties. WHY THE FIGHT ON PESTICIDES IS IMPORTANT All of us are exposed directly or indirectly to pesticides and other agrochemicals- farm workers and their families most of all, but every consumer will be exposed to dozens of different pesti- cides every day through food and the environment. -

Binocular Neuronal Processing of Object Motion in an Arthropod
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Binocular neuronal processing of object motion in an arthropod Florencia Scarano1, Julieta Sztarker1,2, Violeta Medan1,2, Martín Berón de Astrada1,2 and Daniel Tomsic1,2 1Instituto de Fisiología, Biología Molecular y Neurociencias (IFIBYNE) CONICET., Universidad de Buenos Aires, Buenos Aires, Argentina. 2Departamento de Fisiología, Biología Molecular y Celular Dr. Héctor Maldonado., Universidad de Buenos Aires, Facultad de Ciencias Exactas y Naturales. DOI: 10.1523/JNEUROSCI.3641-17.2018 Received: 27 December 2017 Revised: 2 June 2018 Accepted: 5 June 2018 Published: 16 July 2018 Author contributions: F.S., J.S., and D.T. designed research; F.S., V.M., and M.B.d.A. performed research; F.S., J.S., V.M., M.B.d.A., and D.T. analyzed data; F.S., J.S., and D.T. wrote the paper; J.S. and D.T. edited the paper; D.T. wrote the first draft of the paper. Conflict of Interest: The authors declare no competing financial interests. This work was supported by the following grants to DT: PICT 2013--0450 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) and Grant No 20020130100583BA (Universidad de Buenos Aires Ciencia y Tecnología [UBACYT]) from University of Buenos Aires. Corresponding author: Daniel Tomsic. Ciudad Universitaria, Pabellón II, piso 2. FBMC-FCEN, 1428 Buenos Aires. Argentina, e-mail: [email protected] Cite as: J. Neurosci ; 10.1523/JNEUROSCI.3641-17.2018 Alerts: Sign up at www.jneurosci.org/cgi/alerts to receive customized email alerts when the fully formatted version of this article is published. -

Acknowledgements
Acknowledgements We would like to thank those involved in creating Planning a Drift Catcher Project and Organizing a Drift Campaign, including: Jeff Conant from the Hesperian Foundation; Mateo Rutherford and Roy Rojas of BITTS for translation; Brenda J. Willoughby (Pesticide Action Network North America) for layout; and contributors Andrea Wilson and Tracey Brieger (Californians for Pesticide Reform) and Katherine Mills, Susan Kegley, Tanya Brown, Kelly Campbell and Christine Riordan (Pesticide Action Network North America). Major funding for this guide and development of the Drift Catcher was provided by the Cedar Tree Foundation. Additional support was provided by grants to Pesticide Action Network North America and/or Californians for Pesticide Reform by the Beldon Fund, The California Endowment, The California Wellness Foundation, Columbia Foundation, Nathan Cummings Foundation, David B. Gold Foundation, Richard and Rhoda Goldman Foundation, Clarence E. Heller Charitable Foundation, David H. Klein, Jr. Foundation and John Merck Fund. The authors bear responsibility for any factual errors. Recommendations and views expressed are those of Pesticide Action Network North America, and do not necessarily represent the views of our funders and supporters. © 2012 by Pesticide Action Network North America. Permission is granted to reproduce portions of this report, provided the title and publishing organizations—Pesticide Action Network and Californians for Pesticide Reform—are acknowledged. Our sincerest thanks to the Hesperian Foundation for providing many of the images used in these materials. Copyright © 2003 by the Hesperian Foundation. The Hesperian Foundation encourages others to copy, reproduce, or adapt to meet local needs any or all of this pamphlet provided that what is reproduced is distributed free or at cost—not for profit.