Clinical an Urgent Care Approach to Complications and Conditions of Pregnancy Part 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

USMLE – What's It
Purpose of this handout Congratulations on making it to Year 2 of medical school! You are that much closer to having your Doctor of Medicine degree. If you want to PRACTICE medicine, however, you have to be licensed, and in order to be licensed you must first pass all four United States Medical Licensing Exams. This book is intended as a starting point in your preparation for getting past the first hurdle, Step 1. It contains study tips, suggestions, resources, and advice. Please remember, however, that no single approach to studying is right for everyone. USMLE – What is it for? In order to become a licensed physician in the United States, individuals must pass a series of examinations conducted by the National Board of Medical Examiners (NBME). These examinations are the United States Medical Licensing Examinations, or USMLE. Currently there are four separate exams which must be passed in order to be eligible for medical licensure: Step 1, usually taken after the completion of the second year of medical school; Step 2 Clinical Knowledge (CK), this is usually taken by December 31st of Year 4 Step 2 Clinical Skills (CS), this is usually be taken by December 31st of Year 4 Step 3, typically taken during the first (intern) year of post graduate training. Requirements other than passing all of the above mentioned steps for licensure in each state are set by each state’s medical licensing board. For example, each state board determines the maximum number of times that a person may take each Step exam and still remain eligible for licensure. -

HHS Public Access Author Manuscript
HHS Public Access Author manuscript Author Manuscript Author ManuscriptObstet Gynecol Author Manuscript. Author Author Manuscript manuscript; available in PMC 2016 January 12. Published in final edited form as: Obstet Gynecol. 2013 October ; 122(4): 885–900. doi:10.1097/AOG.0b013e3182a5fdfd. Prophylaxis and Treatment of Anthrax in Pregnant Women: A Systematic Review of Antibiotics Dana Meaney-Delman, MD MPH1, Sonja A. Rasmussen, MD MS1, Richard H. Beigi, MD2, Marianne E. Zotti, DrPH1, Yalonda Hutchings, MD MPH1, William A. Bower, MD1, Tracee A. Treadwell, DVM MPH1, and Denise J. Jamieson, MD MPH1 1Centers for Disease Control and Prevention, Atlanta, Georgia 2Department of Obstetrics, Gynecology and Reproductive Sciences, Division of Reproductive Infectious Diseases and Obstetric Specialties, Magee-Women’s Hospital of the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania Abstract Objective—To review the safety and pharmacokinetics of antibiotics recommended for anthrax post-exposure prophylaxis and treatment in pregnant women. Data Sources—Articles were identified in the PUBMED database from inception through December 2012 by searching the keywords ([“pregnancy]” and [generic antibiotic name]). Additionally, hand searches of references from REPROTOX, TERIS, review articles and Briggs’ Drugs in Pregnancy and Lactation were performed. Methods of Study Selection—Articles included in the review contain primary data related to the safety and pharmacokinetics among pregnant women of five antibiotics recommended for anthrax post-exposure prophylaxis and treatment (ciprofloxacin, levofloxacin, moxifloxacin, doxycycline, amoxicillin), and of nine additional antibiotics recommended as part of the treatment regimen (penicillin, ampicillin, linezolid, clindamycin, meropenem, doripenem, rifampin, chloramphenicol, or vancomycin). Tabulation, Integration and Results—The PUBMED search identified 3850 articles for review. -

Effect of Synbiotic on the Treatment of Jaundice in Full Term Neonates: a Randomized Clinical Trial
Pediatr Gastroenterol Hepatol Nutr. 2019 Sep;22(5):453-459 https://doi.org/10.5223/pghn.2019.22.5.453 pISSN 2234-8646·eISSN 2234-8840 Original Article Effect of Synbiotic on the Treatment of Jaundice in Full Term Neonates: A Randomized Clinical Trial Shokoufeh Ahmadipour ,1,2 Parastoo Baharvand ,3 Parisa Rahmani ,4 Amin Hasanvand ,5 and Azam Mohsenzadeh 2 1Razi Herbal Medicine Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran 2Department of Pediatrics, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran 3Department of Social Medicine, School of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran 4Pediatric Gastroenterology and Hepatology Research Center, Tehran University of Medical Sciences, Tehran, Iran 5Department of Pharmacology and Toxicology, Faculty of Pharmacy, Lorestan University of Medical Sciences, Khorramabad, Iran Received: Jun 27, 2018 Revised: Mar 28, 2019 ABSTRACT Accepted: Apr 8, 2019 Purpose: Jaundice accounts for most hospital admissions in the neonatal period. Nowadays, Correspondence to in addition to phototherapy, other auxiliary methods are used to reduce jaundice and the Azam Mohsenzadeh length of hospitalization. This study aimed to investigate the effect of probiotics on the Department of Pediatrics, Faculty of Medicine, Lorestan University of Medical Sciences, treatment of hyper-bilirubinemia in full-term neonates. Anooshirvan Rezaei Square, Khorramabad, Methods: In this randomized clinical trial, 83 full-term neonates, who were admitted to the Lorestan 6813833946, Iran. hospital to receive phototherapy in the first 6 months of 2015, were randomly divided into E-mail: [email protected] two groups: synbiotic (SG, n=40) and control (CG, n=43). Both groups received phototherapy Copyright © 2019 by The Korean Society of but the SG also received 5 drops/day of synbiotics. -

Clinical Relevance of Bleeding Per Vaginam in Early Pregnancy (Before 26 Weeks Gestation) in Patients Recruited from 28Weeks to Delivery
Clinical Relevance of Bleeding Per Vaginam in Early Pregnancy (Before 26 Weeks Gestation) in Patients Recruited from 28weeks to Delivery *Chukwunyere A, *Enaruna N Correspondence Dr. N, Enaruna *Department of Obstetrics and Gynaecology, Department of Obstetrics and Gynaecology, University of Benin Teaching Hospital, Benin University of Benin Teaching Hospital City, Nigeria. Benin City, Edo State Nigeria. E-mail: [email protected] Citation: Chukwunyere A, Enaruna N (2020). Clinical relevance of bleeding per vaginam in early pregnancy (before 26 weeks gestation) in patients recruited from 28weeks to delivery. Nig J Med Dent Educ; 2(2):c28-c31. INTRODUCTION imaging studies are then used for confirmation Bleeding per vaginum in pregnancy is a common (Oguntoyinbo, 2011). presentation in obstetrics. Incidence in literature The objective of this study was to demonstrate ranges from 12% to as high as 40% (Olugbenga, clinical relevance of bleeding per vaginam in early 2019). It can occur in all stages of pregnancy but pregnancy (before 26 weeks gestation). commoner in early pregnancy and has been reported to affect 20% to 30% of pregnancies (Kalyani 2015). MATERIALS AND METHODS The aetiology and source is most times always Women with bleeding per vaginum in early maternal, rather than fetal. Bleeding can result from pregnancy were approached and those who agreed disruption of blood vessels in the decidua or from to participate and gave informed consent were discrete cervical or vaginal lesions (Gupta, 2016; recruited. Information regarding the experience of Tiparse, 2017). Common aetiology includes bleeding in early pregnancy documented and threatened miscarriage, miscarriage, ectopic relevant data on sociodemographic characteristics, gestation and molar gestation. -

Anti-Infective Drug Development in Neonates September 15, 2016
Anti-Infective Drug Development in Neonates September 15, 2016 Page 1 1 FOOD AND DRUG ADMINISTRATION (FDA) 2 3 PUBLIC WORKSHOP: 4 FACILITATING ANTI-INFECTIVE DRUG DEVELOPMENT FOR 5 NEONATES AND YOUNG INFANTS 6 7 September 15, 2016 8 9 10 Sheraton Silver Spring 11 8777 Georgia Avenue 12 Silver Spring, Maryland 20910 13 14 15 16 17 18 19 20 21 Reported by: Chaz Bennett 22 Capital Reporting Company www.CapitalReportingCompany.com 202-857-3376 Anti-Infective Drug Development in Neonates September 15, 2016 Page 2 1 P A R T I C I P A N T S 2 3 John Alexander, MD, MPH 4 Deputy Director 5 Division of Pediatric and Maternal Health (DPMH) 6 Office of Drug Evaluation IV (ODEIV), CDER 7 Food and Drug Administration, Silver Spring, Maryland 8 9 Gerri Baer, MD 10 Medical Officer, Office of Pediatric Therapeutics 11 Food and Drug Administration, Silver Spring, Maryland 12 13 Danny Benjamin, MD, PhD, MPH 14 Kiser-Arena Distinguished Professor of Pediatrics 15 Chair, Pediatric Trials Network 16 Faculty Associate Director, Duke Clinical 17 Research Institute 18 Duke University, Durham, North Carolina 19 20 21 22 www.CapitalReportingCompany.com 202-857-3376 Anti-Infective Drug Development in Neonates September 15, 2016 Page 3 1 P A R T I C I P A N T S 2 (Continued) 3 4 John Bradley, MD 5 Professor and Chief of the Division of Infectious 6 Diseases in the Department of Pediatrics 7 University of California, San Diego 8 9 Maria Fernandez Cortizo 10 PDCU 11 12 John Farley, MD, MPH 13 Deputy Director 14 Office of Antimicrobial Products (OAP), CDER 15 Food and Drug Administration, Silver Spring, Maryland 16 17 William Hope, PhD 18 Professor of Therapeutics and Infectious Diseases 19 University of Liverpool, Liverpool, U.K. -

Ectopic Pregnancy PPT B&W
Early pregnancy bleeding Ectopic pregnancy Dr. Kakali Saha MBBS, FCPS, MS (Obs & Gynae) Associate Professor Medical College for Women & Hospital Ectopic pregnancy • Definition : An ectopic pregnancy is one in which the fertilised ovum becomes implanted in a site other than the normal uterine cavity. • Extrauterine pregnancy -but rudimentary horn of a bicornuate uterus. • It is the consequence of an abnormal implantation of the blastocyst. Incedence • Worldwide 3-4% of all pregnancy. • In USA 2% • Some study 16 in 1000. Past 20 years incidence risen ✦ After one ectopic - there is a7-13 fold increase risk of subsequent ectopic ✦ Subsequent intrauterine preg —50-80% ✦Tubal preg 10-25% ✦Infertile — remaining patient Sites of ectopic pregnancy According to frequency • Fallopian tubes 95-98% (At fimbriated end 17%, Ampulla-55%,Isthmus 25% interstitial 3%) • Uterine cornu 2-2.5% • Ovary, Cervix & abdominal cavity <1% • Right side is more common than left. Risk factors • PID (pelvic inflammatory diseases —6 fold increases risk • Use of IUCD —3-5% increased risks • Smoking 2.5% increased risks • ART 3-5% increased risks • Tubal damage • Tubal surgery 5.8% • Salpingitis isthmica nodes 3.5% increased risks • Prior ectopic pregnancy cont. Risk factor • Age 3 fold increased risks in 35-44 years compared to 18 -24 yrs • Non white race 1.5 fold increased risks • Endometriosis 1.5 increased risks • Developmental errors • Overdevelopment of ovum & external migration . Aetiology • Tubal damage or altered motility results improper transport of blastocyst • Most common cause is acute salpingitis 50% • In 40% no risk factors apparent • Salpingitis causes peritubal adhesion , lumen occlusion , intratubal adhesion diverticula & disturbed tubal function. -

Iatrogenic Complications in the Neonatal Intensive Care Unit
Journal of Perinatology (2010) 30, S51–S56 r 2010 Nature America, Inc. All rights reserved. 0743-8346/10 www.nature.com/jp REVIEW Iatrogenic complications in the neonatal intensive care unit KC Sekar Department of Pediatrics, Neonatal-Perinatal Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA In neonatal medicine, significant iatrogenic events leading With the introduction of novel technologies and approaches in neonatal to severe morbidity and death have been identified and addressed. care and the lack of appropriately designed and well-executed randomized Low incubator temperature leading to death (1930); irradiation clinical trials to investigate the impact of these interventions, iatrogenic causing thyroid cancer, and excess oxygen exposure causing complications have been increasingly seen in the neonatal intensive care retinopathy of prematurity and blindness (1940); excess unit. In addition, increased awareness and the introduction of more synthetic vitamin K, sulfisoxazole and chloramphenicol causing appropriate quality control measures have resulted in higher levels of kernicterus and gray baby syndrome (1950); intrauterine suspicion about and increased recognition of complications associated with exposure to thalidomide causing limb defects (1960); umbilical delivery of care. The incidence of complications also rises with the increased arterial catheter, tolazoline and total parenteral nutrition (TPN) length of hospital stay and level of immaturity. Approximately half of the causing thrombosis, hypotension and essential fatty acid deficiency, iatrogenic complications are related to medication errors. The other respectively (1970); vitamin E and propylene glycol causing complications are due to nosocomial infections, insertion of invasive organ damage, hyperosmolarity and seizures (1980); and catheters, prolonged mechanical ventilation, administration of parenteral mechanical ventilation and systemic corticosteroid administration nutrition solution, skin damage and environmental complications. -

Learning Objectives: 1. Relation of Drugs and Lactation 2. Factors Modifying Passage of Drugs in Milk 3
Learning Objectives: 1. Relation of drugs and lactation 2. Factors modifying passage of drugs in milk 3. Effects of drugs on milk production 4. Role of lactation on drugs excretion This lecture was done by: 5. Drug safety during lactation / use of Abdullah Saleh Bawazir safe drugs 6. Drugs contraindicated during lactation And reviewed by: Tuqa Alkaff Lactaon: • Breast feeding is very important because breast milk is the healthiest form of milk for babies. • It provides the baby with immunoglobulins (IgA, IgM) that are essenAal for protecAon against gastroenteriAs. Drugs & Lactaon: . Most drugs administered to breast feeding woman are detectable in milk. The concentraon of drugs achieved in breast milk is usually low (< 1 %). However, even small amounts of some drugs may be of significance for the suckling child. (all the execratory mechanisms are going down in babies) Pediatric populaon are classified into: • Newborn: less than one month old – Preterm neonates: born before 38 weeks of pregnancy (it's the harmful stage) – Full-term neonates: 38-42 weeks of gestaonal age • Infants (babies): 1 month – 12 months of age • Children: 1 -12 years of age – Toddler (young child): 1-5 years – Older child: 6-12 years • Adolescent: 13-18 years 2 Pharmacokine%cs in pediatric: • Higher gastric PH • Higher concentraon of free drug • Higher percenrage of body water • Neonate’s especially premature babies have limited capacity for metabolism and excreAon. • Neonates have very limited rate of metabolism due to immaturity of liver enzymes. • Renal clearance is less efficient: ( decrease Renal blood flow – decrease GFR). • The epithelium of the breast alveolar cells is most permeable to drugs during the 1st week postpartum, so drug transfer to milk may be greater during the 1st week of an infant’s life. -
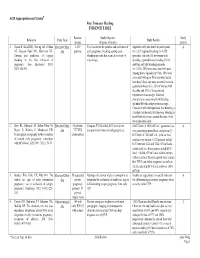
ACR Appropriateness Criteria® First Trimester Bleeding EVIDENCE TABLE
ACR Appropriateness Criteria® First Trimester Bleeding EVIDENCE TABLE Patients/ Study Objective Study Reference Study Type Study Results Events (Purpose of Study) Quality 1. Hasan R, Baird DD, Herring AH, Olshan Review/Other 4,539 To characterize the patterns and predictors of Approximately one-fourth of participants 4 AF, Jonsson Funk ML, Hartmann KE. -Dx patients early pregnancy bleeding, setting aside (n=1,207) reported bleeding (n=1,656 Patterns and predictors of vaginal bleeding episodes that occur at the time of episodes), but only 8% of women with bleeding in the first trimester of miscarriage. bleeding, reported heavy bleeding. Of the pregnancy. Ann Epidemiol 2010; spotting and light bleeding episodes 20(7):524-531. (n=1,555), 28% were associated with pain. Among heavy episodes (n=100), 54% were associated with pain. Most episodes lasted less than 3 days, and most occurred between gestational weeks 5–8. 12% of women with bleeding and 13% of those without experienced miscarriage. Maternal characteristics associated with bleeding included fibroids and prior miscarriage. Consistent with the hypothesis that bleeding is a marker for placental dysfunction, bleeding is most likely to be seen around the time of the luteal-placental shift. 2. Bree RL, Edwards M, Bohm-Velez M, Review/Other 53 patients; Compare TVUS with -hCG level in the -hCG level of 1000 mIU/ml - gestational sac 4 Beyler S, Roberts J, Mendelson EB. -Dx 75 TVUS evaluation of embryo in early pregnancy. was seen sonographically in each patient. - Transvaginal sonography in the evaluation examinations hCG level of 7200 mIU/ml - yolk sac was of normal early pregnancy: correlation seen in every patient. -

Is Pediatric Labeling Really Necessary?
Is Pediatric Labeling Really Necessary? Michael L. Christensen, PharmD; Richard A. Helms, PharmD; and Russell W. Chesney, MD ABBREVIATIONS. FDA, US Food and Drug Administration; For solid dosage forms, parents often must divide FDCA, Food, Drug and Cosmetic Act; GFR, glomerular filtration adult tablets in halves or quarters to get an appro- rate; BSA, body surface area. priate dose for their child. This results in imprecise dosing that can lead to poor therapeutic response. abeling refers to the label on the drug container Practitioners are left to use empiric therapy in and all printed materials, including the pack- treating their pediatric patients because of the inad- age insert, that accompanies the product. La- equate information available for prescribing of L drugs. They often must choose between not treating beling of a drug indicates that there is substantial evidence from adequate and well controlled clinical children with potentially beneficial medications be- trials for the safe and effective use of that drug. cause they are not approved for use in children, or to Labeling provides important information on clinical treat them with these medications based on adult pharmacology, indications and usage, contraindica- studies and limited or anecdotal experience in chil- tions, precautions, adverse effects, dosage, and ad- dren. In either case, children may not be prescribed ministration. Unfortunately for children, most drug optimal therapy. labeling contains the precautionary disclaimer, be- cause safety and efficacy in children have not been FOOD, DRUG AND COSMETIC ACT established. Concern for the risk to public health and safety The availability of safe and effective drugs has with unsanitary food and adulterated drugs, a com- been directly responsible for the improvement in mon problem during that time, lead to passage of health over the past 50 years. -

Abortus, 4 Absolute Neutrophil Count, 146 Absorption
Index A on respiratory syncytial virus, 158, 160 on sedation management, 112 Abortus, 4 on systemic corticosteroids, 88 Absolute neutrophil count, 146 on vaccinations, 187 Absorption, 17 on Vitamin K IM, 10-11 extravascular, 18 American College of Chest Physicians, 159 gastrointestinal, 18 American College of Emergency Physicians, 159 intramuscular, 18 American College of Obstetrics and Gynecology, 61 percutaneous, 19 American Congress of Obstetricians and rectal, 19 Gynecologists, 145 Academy of Breastfeeding Medicine, 49, 61 American Society for Parenteral and Enteral Acetaminophen, 21, 119, 126 Nutrition, 30, 34 PDA and, 103-104 American Society of Health-System Pharmacists vaccination response and, 191-192 (ASHP), 34 Acid ionization constant, breast milk and, 47 American Thoracic Society, 159 Acid-base balance, 32 Amino acids, 30, 34-35 Activity, 5 Aminoglycoside(s), 19, 134, 172 Acute lung injury, 84-85 Aminophylline, 69 Acyclovir, 46 Aminosyn, 30 parenteral, 147-148 Amoxicillin-sulbactam, 172 Addiction, maternal, 61 Amphotericin B, 137 Adverse drug events, methylxanthine toxicity, 71 Ampicillin, 6, 134, 172 Adverse effects, neonatal abstinence treatment, 60 Ampicillin-sulbactam, 172 Airway patency maintenance, 68 Anaerobic antibiotic therapy, 172 Albuterol, 87, 161 Analgesia Alcohol, 48 common agents for, 127-129 Alginate, 181 pharmacologic agents for, 118-119 Alpha agonists, 115-116 principles of, 116-117 Aluminum toxicity, 36-37 Analgesics, 123 American Academy of Pediatrics, 45 reversal of, 121 acyclovir shortage recommendations, -

Evidence of Drug Safety in Pregnancy
DRUGS IN PREGNANCY Evidence of drug safety in pregnancy For an area as complex as prescribing in pregnancy, unfortunately, there is very little information in the literature to support practice. Newer antimicrobials are trialled excluding pregnant women for reasons of risk-avoidance and, therefore, prescribers must rely on post-marketing surveillance data. For some medications, this takes the form of a formal registry (for example, the antiretroviral pregnancy registry). Obviously, prospective safety data from Phase I/II clinical trials is preferable, but in the absence of this, registry data provides some reassurance. Older antibiotics, on the other hand, may have many years of practical experience to suggest their safety, but little robust scientific evidence to support their safe use. Evidence of treatment efficacy in pregnancy Large prospective trials of treatment in pregnancy are conspicuously absent from the literature. There have been a number of Cochrane reviews on different topics in the treatment of infections in pregnancy – from asymptomatic bacteriuria to bacterial vaginosis. All of the reviews have in common very heterogeneous primary articles and cautious recommendations based on lack of data. In general, antibiotics are effective at treating the infection in question, but studies are underpowered to provide information on optimum therapy or fetal safety. 1 Done by : Pharm.D –Neda'Rwashdeh Infection and pregnancy Immune tolerance’ in pregnancy is widely discussed and there are some data to suggest that pregnant women are at slightly higher risk of developing disease from some infections (including poliomyelitis, smallpox, hepatitis A and falciparum malaria). Also, the risk of severe disease and death are increased for some infections.