12-Hydroxyheptadecatrienoic Acid Promotes Epidermal Wound Healing by Accelerating Keratinocyte Migration Via the BLT2 Receptor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Serum Malondialdehyde Is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women
antioxidants Article Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women Shira Zelber-Sagi 1,2,*, Dana Ivancovsky-Wajcman 1, Naomi Fliss-Isakov 2,3, Michal Hahn 4, 2,3 2,3 2,3, 4, Muriel Webb , Oren Shibolet , Revital Kariv y and Oren Tirosh y 1 School of Public Health, University of Haifa, Haifa 3498838, Israel; [email protected] 2 Department of Gastroenterology, Tel Aviv Medical Center, Tel Aviv 6423914, Israel; naomifl@tlvmc.gov.il (N.F.-I.); [email protected] (M.W.); [email protected] (O.S.); [email protected] (R.K.) 3 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 4 Institute of Biochemistry, Food Science and Nutrition, The RH Smit Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rechovot 76100001, Israel; [email protected] (M.H.); [email protected] (O.T.) * Correspondence: [email protected]; Tel.: +972-3-6973984 The last two authors equally contributed to the paper. y Received: 25 May 2020; Accepted: 25 June 2020; Published: 2 July 2020 Abstract: Background: Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) are associated with increased oxidative stress and lipid peroxidation, but large studies are lacking. The aim was to test the association of malondialdehyde (MDA), as a marker of oxidative damage of lipids, with NAFLD and liver damage markers, and to test the association between dietary vitamins E and C intake and MDA levels. Methods: A cross-sectional study was carried out among subjects who underwent blood tests including FibroMax for non-invasive assessment of NASH and fibrosis. -

Oxisresearch™ a Division of OXIS Health Products, Inc
OxisResearch™ A Division of OXIS Health Products, Inc. BIOXYTECHÒ MDA-586™ Spectrophotometric Assay for Malondialdehyde For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number 21044 INTRODUCTION The Analyte Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals, and is used as an indicator of oxidative stress in cells and tissues. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds. These include reactive carbonyl compounds, of which the most abundant is malondialdehyde (MDA). Therefore, measurement of malondialdehyde is widely used as an indicator of lipid peroxidation (1). Increased levels of lipid peroxidation products have been associated with a variety of chronic diseases in both humans (2, 3) and model systems (4, 5). MDA reacts readily with amino groups on proteins and other biomolecules to form a variety of adducts (1), including cross-linked products (6). MDA also forms adducts with DNA bases that are mutagenic (7, 8) and possibly carcinogenic (9). DNA-protein cross-links are another result of the reaction between DNA and MDA (10). The TBARS method is commonly used to measure MDA in biological samples (11). However, this reaction is relatively nonspecific; both free and protein-bound MDA can react. The MDA-586 method is designed to assay free MDA or, after a hydrolysis step, total MDA (i.e., free and protein-bound Schiff base conjugates). The assay conditions serve to minimize interference from other lipid peroxidation products, such as 4- hydroxyalkenals. PRINCIPLES OF THE PROCEDURE The MDA-586 method1 (12) is based on the reaction of a chromogenic reagent, N-methyl-2- phenylindole (R1, NMPI), with MDA at 45°C. -

Neuroprotective Effects of Geniposide from Alzheimer's Disease Pathology
Neuroprotective effects of geniposide from Alzheimer’s disease pathology WeiZhen Liu1, Guanglai Li2, Christian Hölscher2,3, Lin Li1 1. Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China 2. Second hospital, Shanxi medical University, Taiyuan, PR China 3. Neuroscience research group, Faculty of Health and Medicine, Lancaster University, Lancaster LA1 4YQ, UK running title: Neuroprotective effects of geniposide corresponding author: Prof. Lin Li Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China Email: [email protected] Neuroprotective effects of geniposide Abstract A growing body of evidence have linked two of the most common aged-related diseases, type 2 diabetes mellitus (T2DM) and Alzheimer disease (AD). It has led to the notion that drugs developed for the treatment of T2DM may be beneficial in modifying the pathophysiology of AD. As a receptor agonist of glucagon- like peptide (GLP-1R) which is a newer drug class to treat T2DM, Geniposide shows clear effects in inhibiting pathological processes underlying AD, such as and promoting neurite outgrowth. In the present article, we review possible molecular mechanisms of geniposide to protect the brain from pathologic damages underlying AD: reducing amyloid plaques, inhibiting tau phosphorylation, preventing memory impairment and loss of synapses, reducing oxidative stress and the chronic inflammatory response, and promoting neurite outgrowth via the GLP-1R signaling pathway. In summary, the Chinese herb geniposide shows great promise as a novel treatment for AD. Key words: Alzheimer’s disease, geniposide, amyloid-β, neurofibrillary tangles, oxidative stress, inflammatation, type 2 diabetes mellitus, glucagon like peptide receptor, neuroprotection, tau protein Neuroprotective effects of geniposide 1. -

Phenethylamine in Chlorella Alleviates High-Fat Diet-Induced Mouse Liver
www.nature.com/npjscifood ARTICLE OPEN Phenethylamine in chlorella alleviates high-fat diet-induced mouse liver damage by regulating generation of methylglyoxal ✉ Yifeng Zheng1, Agustin Martin-Morales1, Jing Wang1, Masaki Fujishima 2, Eri Okumura 2 and Kenji Sato 1 This study examined the effects of oral administration of water extract of chlorella (WEC) (100 mg/kg bodyweight) and phenethylamine (10 μg/kg bodyweight) on high-fat diet (HFD)-induced liver damage in mice. Phenethylamine significantly mitigated HFD-induced lipid oxidation (generation of malondialdehyde) and liver damage without markedly decreasing hepatic lipid accumulation. WEC exerted similar effects although with decreased efficacy. In addition, WEC and phenethylamine decreased the methylglyoxal levels and increased the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels in the liver. Methylglyoxal is generated from substrates of GAPDH, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. These facts indicate that methylglyoxal triggers oxidation of accumulated lipid, which generates malondialdehyde and consequently induces liver damage. Suppression of generation of toxic aldehydes by WEC and phenethylamine was also confirmed by maintaining hepatic cysteine, highly reactive to aldehydes. Thus, trace amounts of phenethylamine alleviate HFD-induced liver damage by regulating methylglyoxal via increase of GAPDH. npj Science of Food (2021) 5:22 ; https://doi.org/10.1038/s41538-021-00105-3 1234567890():,; INTRODUCTION lipid deposition-induced oxidative stress in the liver contributes to Chlorella pyrenoidosa, a freshwater unicellular green alga, and its the pathogenesis of NAFLD13,14, whereas the liver contains potent water extract have a long history of usage as food supplements. antioxidant enzyme systems, such as the superoxide dismutase Various animal studies and clinical trials have reported that C. -

Protective Effects of Geniposide and Genipin Against Hepatic Ischemia/Reperfusion Injury in Mice
Original Article Biomol Ther 21(2), 132-137 (2013) Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice Joonki Kim1, Hyo-Yeon Kim2 and Sun-Mee Lee2,* 1Natural Medicine Center, Korea Institute of Science & Technology, Gangneung 210-340, 2School of Pharmacy, Sungkyunkwan University, Suwon 440-746, Republic of Korea Abstract Geniposide is an active product extracted from the gardenia fruit, and is one of the most widely used herbal preparations for liver disorders. This study examined the cytoprotective properties of geniposide and its metabolite, genipin, against hepatic ischemia/ reperfusion (I/R) injury. C57BL/6 mice were subjected to 60 min of ischemia followed by 6 h of reperfusion. Geniposide (100 mg/ kg) and genipin (50 mg/kg) were administered orally 30 min before ischemia. In the I/R mice, the levels of serum alanine amino- transferase and hepatic lipid peroxidation were elevated, whereas hepatic glutathione/glutathione disulfide ratio was decreased. These changes were attenuated by geniposide and genipin administration. On the other hand, increased hepatic heme oxygen- ase-1 protein expression was potentiated by geniposide and genipin administration. The increased levels of tBid, cytochrome c protein expression and caspase-3 activity were attenuated by geniposide and genipin. Increased apoptotic cells in the I/R mice were also significantly reduced by geniposide and genipin treatment. Our results suggest that geniposide and genipin offer signifi- cant hepatoprotection against I/R injury by reducing oxidative stress and apoptosis. Key Words: Geniposide, Genipin, Ischemia, Reperfusion, Liver, Apoptosis INTRODUCTION 2004). Oral administration of geniposide increased hepatic glutathione (GSH) content, which is responsible for hepatopro- Ischemia/reperfusion (I/R) injury to the liver is of clinical im- tection against aflatoxin B1-induced liver injury in rats (Kanget portance in humans after hemorrhagic and cardiogenic shock, al., 1997). -

Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders
Review Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders Giuseppina Barrera 1,*, Stefania Pizzimenti 1, Martina Daga 1, Chiara Dianzani 2, Alessia Arcaro 3, Giovanni Paolo Cetrangolo 3, Giulio Giordano 4, Marie Angele Cucci 1, Maria Graf 4 and Fabrizio Gentile 3 1 Dipartimento di Scienze Cliniche e Biologiche, Università di Torino, 10124 Turin, Italy; [email protected] (S.P.); [email protected] (M.D.); [email protected] (M.A.C.) 2 Dipartimento di Scienze e Tecnologia del Farmaco, Università di Torino, 10124 Turin, Italy; [email protected] 3 Dipartimento di Medicina e Scienze della Salute “V. Tiberio”, Università del Molise, 86100 Campobasso, Italy; [email protected] (A.A.); [email protected] (G.P.C.); [email protected] (F.G.) 4 Presidio Ospedaliero “A. Cardarelli”, Azienda Sanitaria Regione Molise, 86100 Campobasso, Italy; [email protected] (G.G.); [email protected] (M.G.) * Correspondence: [email protected] Received: 29 June 2018; Accepted: 27 July 2018; Published: 30 July 2018 Abstract: Among the various mechanisms involved in aging, it was proposed long ago that a prominent role is played by oxidative stress. A major way by which the latter can provoke structural damage to biological macromolecules, such as DNA, lipids, and proteins, is by fueling the peroxidation of membrane lipids, leading to the production of several reactive aldehydes. Lipid peroxidation-derived aldehydes can not only modify biological macromolecules, by forming covalent electrophilic addition products with them, but also act as second messengers of oxidative stress, having relatively extended lifespans. Their effects might be further enhanced with aging, as their concentrations in cells and biological fluids increase with age. -

276.Full.Pdf
[CANCER RESEARCH 40, 276-282, February 19801 0008-5472/80/0040-0000$02.00 Comparison of the Mutagenicities of Malondialdehyde and the Side Products Formed during Its Chemical Synthesis1 Lawrence J. Marnett2 and Melissa A. Tuttle Department of Chemistry, Wayne State University. Detroit, Michigan 48202 ABSTRACT (9, 15). It is believed that the formation of these polymers can be avoided by using very dilute solutions of TEP or TMP for the Malondialdehyde, a product of polyunsaturated fatty acid hydrolyses (15). Since MDA is a relatively weak mutagen, high metabolism and degradation, has been reported to be muta concentrations must be used to detect a mutagenic response genic and carcinogenic. The malondialdehyde used for testing (16, 21). This necessitates the use of concentrated solutions was generated by the acidic hydrolysis of tetraalkoxypropanes. of TEP or TMP for the preparation of the MDA solutions. We have studied the production of compounds mutagenic to MDA is a moderatelyweak acid (pK@,,4.46)which exists as Sa!mone!!a typhimurium strain his D 3052 following the hy its conjugate base at physiological pH (Ref. 17; Equation A). drolysis of tetraalkoxypropanes. The major mutagenic com pound produced from tetraethoxypropane is 18-ethoxy-acrolein 0 0 (90 to 100 revertants4tmol) and not malondialdehyde (3 to 5 II @ revertants/jsmol). Hydrolysis of tetramethoxypropane pro H )@ H pKa duces two compounds, $-methoxy-acrolein (125 to 160 re _________ H@ ,,C@ vertants4tmol) and 3,3-dimethoxypropionaldehyde (105 to II 135 revertants/@mol), which are more mutagenic than is ma ,C@ @ londialdehyde. Using standard conditions for the hydrolysis of H H tetraethoxypropane, the yield of malondialdehyde is 25%, and the yield of ,6-ethoxyacrolein is 13%. -
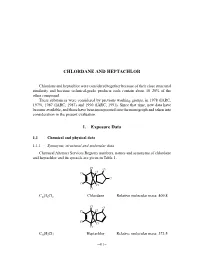
Chlordane and Heptachlor
CHLORDANE AND HEPTACHLOR Chlordane and heptachlor were considered together because of their close structural similarity and because technical-grade products each contain about 10–20% of the other compound. These substances were considered by previous working groups, in 1978 (IARC, 1979), 1987 (IARC, 1987) and 1990 (IARC, 1991). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Chemical Abstract Services Registry numbers, names and synonyms of chlordane and heptachlor and its epoxide are given in Table 1. Cl Cl Cl Cl Cl Cl Cl Cl C10H6Cl8 Chlordane Relative molecular mass: 409.8 Cl Cl Cl Cl Cl Cl Cl C10H5Cl7 Heptachlor Relative molecular mass: 373.5 –411– 412 IARC MONOGRAPHS VOLUME 79 Table 1. Chemical Abstract Services Registry numbers, names and synonyms of chlordane, heptachlor and its epoxide Name CAS Reg. Nosa Chem. Abstr. namesb and synonyms Chlordane 57-74-9 ENT 9932; 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a- (39400-80-1); hexahydro-4,7-methano-1H-indene; 1,2,4,5,6,7,8,8- 53637-13-1) octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano- indene (IUPAC); octachloro-4,7-methanotetrahydro- indane; 1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetra- hydro-4,7-methanoindan; OMS 1437 Technical-grade 12789-03-6 chlordane cis-Chlordane 5103-71-9 α-Chlordan; α-chlordane; cis-chlordan; (152322-29-7; (1α,2α,3aα,4β,7β,7aα)-1,2,4,5,6,7,8,8-octachloro- 22212-52-8; -

The Reactive Carbonyl Derivatives of Proteins, Methylglyoxal, and Malondialdehyde in Blood of Women with Breast Cancer
Scientific Foundation SPIROSKI, Skopje, Republic of Macedonia Open Access Macedonian Journal of Medical Sciences. 2021 Jul 02; 9(B):509-514. https://doi.org/10.3889/oamjms.2021.5564 eISSN: 1857-9655 Category: B - Clinical Sciences Section: Oncology The Reactive Carbonyl Derivatives of Proteins, Methylglyoxal, and Malondialdehyde in Blood of Women with Breast Cancer Sabina Zhumakayeva* , Larissa Muravlyova, Valentina Sirota, Vilen Molotov-Luchansky, Ryszhan Bakirova, Nailya Kabildina, Xeniya Mkhitaryan , Zhumakayeva Ainura Department of Oncology and Radiation Diagnostics, School of Medicine, Karaganda Medical University, Karaganda, Kazakhstan Abstract Edited by: Ksenija Bogoeva-Kostovska BACKGROUND: Every year 1.5 million women in the world are diagnosed with breast cancer (BC). In 2018, more Citation: Zhumakayeva S, Muravlyova L, Sirota V, Molotov-Luchansky V, Bakirova R, Kabildina N, than 260,000 new cases of cancer and more than 40,000 deaths due to this disease were detected. At the same Mkhitaryan X, Ainura Z. The Reactive Carbonyl Derivatives time, in Kazakhstan, an intensive indicator of the incidences of BC in 2018 amounted to 25.3% per population of 100 of Proteins, Methylglyoxal and Malondialdehyde in Blood thousand people (2017–24.5%) with a growth rate of 3.1%, which in absolute numbers are 4,648 new cases per year. of Women with Breast Cancer. Open Access Maced J Med Sci. 2021 Jul 02; 9(B):509-514. In terms of mortality, BC ranks third after lung and stomach cancer (6.8%). https://doi.org/10.3889/oamjms.2021.5564 Keywords: Breast cancer; Oxidative AIM: This necessitates a detailed study of the molecular mechanisms that underlie the development and progression stress; Malondialdehyde of BC. -

Neuroprotective Effects of Geniposide on Alzheimer's Disease Pathology
Rev. Neurosci. 2015; 26(4): 371–383 WeiZhen Liu, Guanglai Li, Christian Hölscher and Lin Li* Neuroprotective effects of geniposide on Alzheimer’s disease pathology Abstract: A growing body of evidence has linked two of Introduction the most common aged-related diseases: type 2 diabe- tes mellitus (T2DM) and Alzheimer’s disease (AD). It has Alzheimer’s disease (AD) is the most common neurode- led to the notion that drugs developed for the treatment generative disorder of progressive cognitive decline in of T2DM may be beneficial in modifying the pathophysi- the aged population. The characteristic pathological hall- ology of AD. As a receptor agonist of glucagon-like pep- marks are the abundance of two abnormal aggregated tide-1 (GLP-1R), which is a newer drug class to treat T2DM, proteins in brain tissue: neurofibrillary tangles (NFTs) geniposide shows clear effects in inhibiting pathologi- composed mainly of the microtubule-associated protein τ cal processes underlying AD, such as promoting neurite and amyloid plaques composed of insoluble amyloid-β outgrowth. In the present article, we review the possi- (Aβ) deposits, synaptic and neuronal loss, as well as ble molecular mechanisms of geniposide to protect the dysfunction associated to the neurochemical changes in brain from pathologic damages underlying AD: reducing brain tissue (Mathis et al., 2007). The multiple molecular amyloid plaques, inhibiting τ phosphorylation, prevent- pathogenic changes contributing to the pathological hall- ing memory impairment and loss of synapses, reducing marks of AD include mitochondrial dysfunction, oxidative oxidative stress and the chronic inflammatory response, stress, endoplasmic reticulum (ER) stress, and inflam- and promoting neurite outgrowth via the GLP-1R signaling mation, which lead to the varying levels of plaques and pathway. -

Findings in Gulf War Illness and Healthy Controls
Golomb et al. Military Med Res (2021) 8:46 https://doi.org/10.1186/s40779-021-00337-0 RESEARCH Open Access Lower blood malondialdehyde is associated with past pesticide exposure: fndings in Gulf War illness and healthy controls Beatrice Alexandra Golomb1* , Sridevi Devaraj2, Alexis K. Messner1,3, Hayley Jean Koslik1,4, Jun Hee Han1 and Barnabas Yik1,5 Abstract Background: Malondialdehyde (MDA) is a candidate general marker of oxidative stress (OS). We sought to assess the relation of MDA to Gulf War illness (GWI) and to a variety of exposures. Methods: This is an observational study involving subjects from Southern California recruited from October 2011 to May 2014. MDA was assessed in 81 participants (41 GWI-cases, 40 controls). General and Gulf-specifc exposures were elicited. MDA case–control comparison was restricted to 40 matched pairs. The potential association between MDA and exposures was assessed using regression analyses. Gulf-specifc exposures were incorporated into a case-specifc model. Results: Plasma MDA was signifcantly lower in GWI-cases than controls. Composite pesticide and fuel-solvent exposures negatively predicted MDA in the total sample, as well as in the analyses that included either GWI-cases or controls only. Self-reported exposure to organophosphate (OP) nerve gas was a strong predictor for lower MDA level in veterans with GWI. Conclusion: Past pesticide exposures predicted lower MDA in both veterans with GWI and in healthy controls. Keywords: Malondialdehyde, Oxidative stress, Free radical, Gulf War veterans, Gulf War illness, Pesticide Background past, in diferent settings and within groups with other Malondialdehyde (MDA) is a measure of peroxidized signatures indicative of OS injury. -

Reactive Carbonyl Compounds (Rccs) Cause Aggregation and Dysfunction of Fibrinogen
Protein Cell 2012, 3(8): 627–640 DOI 10.1007/s13238-012-2057-y Protein & Cell RESEARCH ARTICLE Reactive carbonyl compounds (RCCs) cause aggregation and dysfunction of fibrinogen Ya-Jie Xu1*, Min Qiang1,2*, Jin-Ling Zhang1, Ying Liu1, Rong-Qiao He1,3 1 State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China 2 Graduate University of Chinese Academy of Sciences, Beijing 100049, China 3 Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China Correspondence: [email protected] Received June 5, 2012 Accepted July 1, 2012 ABSTRACT pathological process for fibrinogen (fibrin) deposition in the blood. Fibrinogen is a key protein involved in coagulation and its deposition on blood vessel walls plays an important KEYWORDS fibrinogen, acrolein, glycolaldehyde, role in the pathology of atherosclerosis. Although the glyoxal, malondialdehyde, methylglyoxal causes of fibrinogen (fibrin) deposition have been studied in depth, little is known about the relationship INTRODUCTION between fibrinogen deposition and reactive carbonyl compounds (RCCs), compounds which are produced Fibrinogen is a key protein involved in blood coagulation and and released into the blood and react with plasma hemostasis (Liu et al., 1979; Phillips et al., 1988; Sidelmann protein especially under conditions of oxidative stress et al., 2000). Both the amount (physiological concentrations and inflammation. Here, we investigated the effect of normally range from 1.5 mg/mL to 4 mg/mL in human blood) glycolaldehyde on the activity and deposition of and the activity of fibrinogen are important for maintaining the fibrinogen compared with the common RCCs acrolein, equilibrium of coagulation (Kamath and Lip, 2003).