HNE) in the Balance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Serum Malondialdehyde Is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women
antioxidants Article Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women Shira Zelber-Sagi 1,2,*, Dana Ivancovsky-Wajcman 1, Naomi Fliss-Isakov 2,3, Michal Hahn 4, 2,3 2,3 2,3, 4, Muriel Webb , Oren Shibolet , Revital Kariv y and Oren Tirosh y 1 School of Public Health, University of Haifa, Haifa 3498838, Israel; [email protected] 2 Department of Gastroenterology, Tel Aviv Medical Center, Tel Aviv 6423914, Israel; naomifl@tlvmc.gov.il (N.F.-I.); [email protected] (M.W.); [email protected] (O.S.); [email protected] (R.K.) 3 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 4 Institute of Biochemistry, Food Science and Nutrition, The RH Smit Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rechovot 76100001, Israel; [email protected] (M.H.); [email protected] (O.T.) * Correspondence: [email protected]; Tel.: +972-3-6973984 The last two authors equally contributed to the paper. y Received: 25 May 2020; Accepted: 25 June 2020; Published: 2 July 2020 Abstract: Background: Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) are associated with increased oxidative stress and lipid peroxidation, but large studies are lacking. The aim was to test the association of malondialdehyde (MDA), as a marker of oxidative damage of lipids, with NAFLD and liver damage markers, and to test the association between dietary vitamins E and C intake and MDA levels. Methods: A cross-sectional study was carried out among subjects who underwent blood tests including FibroMax for non-invasive assessment of NASH and fibrosis. -

Oxisresearch™ a Division of OXIS Health Products, Inc
OxisResearch™ A Division of OXIS Health Products, Inc. BIOXYTECHÒ MDA-586™ Spectrophotometric Assay for Malondialdehyde For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number 21044 INTRODUCTION The Analyte Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals, and is used as an indicator of oxidative stress in cells and tissues. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds. These include reactive carbonyl compounds, of which the most abundant is malondialdehyde (MDA). Therefore, measurement of malondialdehyde is widely used as an indicator of lipid peroxidation (1). Increased levels of lipid peroxidation products have been associated with a variety of chronic diseases in both humans (2, 3) and model systems (4, 5). MDA reacts readily with amino groups on proteins and other biomolecules to form a variety of adducts (1), including cross-linked products (6). MDA also forms adducts with DNA bases that are mutagenic (7, 8) and possibly carcinogenic (9). DNA-protein cross-links are another result of the reaction between DNA and MDA (10). The TBARS method is commonly used to measure MDA in biological samples (11). However, this reaction is relatively nonspecific; both free and protein-bound MDA can react. The MDA-586 method is designed to assay free MDA or, after a hydrolysis step, total MDA (i.e., free and protein-bound Schiff base conjugates). The assay conditions serve to minimize interference from other lipid peroxidation products, such as 4- hydroxyalkenals. PRINCIPLES OF THE PROCEDURE The MDA-586 method1 (12) is based on the reaction of a chromogenic reagent, N-methyl-2- phenylindole (R1, NMPI), with MDA at 45°C. -

Neuroprotective Effects of Geniposide from Alzheimer's Disease Pathology
Neuroprotective effects of geniposide from Alzheimer’s disease pathology WeiZhen Liu1, Guanglai Li2, Christian Hölscher2,3, Lin Li1 1. Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China 2. Second hospital, Shanxi medical University, Taiyuan, PR China 3. Neuroscience research group, Faculty of Health and Medicine, Lancaster University, Lancaster LA1 4YQ, UK running title: Neuroprotective effects of geniposide corresponding author: Prof. Lin Li Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, PR China Email: [email protected] Neuroprotective effects of geniposide Abstract A growing body of evidence have linked two of the most common aged-related diseases, type 2 diabetes mellitus (T2DM) and Alzheimer disease (AD). It has led to the notion that drugs developed for the treatment of T2DM may be beneficial in modifying the pathophysiology of AD. As a receptor agonist of glucagon- like peptide (GLP-1R) which is a newer drug class to treat T2DM, Geniposide shows clear effects in inhibiting pathological processes underlying AD, such as and promoting neurite outgrowth. In the present article, we review possible molecular mechanisms of geniposide to protect the brain from pathologic damages underlying AD: reducing amyloid plaques, inhibiting tau phosphorylation, preventing memory impairment and loss of synapses, reducing oxidative stress and the chronic inflammatory response, and promoting neurite outgrowth via the GLP-1R signaling pathway. In summary, the Chinese herb geniposide shows great promise as a novel treatment for AD. Key words: Alzheimer’s disease, geniposide, amyloid-β, neurofibrillary tangles, oxidative stress, inflammatation, type 2 diabetes mellitus, glucagon like peptide receptor, neuroprotection, tau protein Neuroprotective effects of geniposide 1. -

Phenethylamine in Chlorella Alleviates High-Fat Diet-Induced Mouse Liver
www.nature.com/npjscifood ARTICLE OPEN Phenethylamine in chlorella alleviates high-fat diet-induced mouse liver damage by regulating generation of methylglyoxal ✉ Yifeng Zheng1, Agustin Martin-Morales1, Jing Wang1, Masaki Fujishima 2, Eri Okumura 2 and Kenji Sato 1 This study examined the effects of oral administration of water extract of chlorella (WEC) (100 mg/kg bodyweight) and phenethylamine (10 μg/kg bodyweight) on high-fat diet (HFD)-induced liver damage in mice. Phenethylamine significantly mitigated HFD-induced lipid oxidation (generation of malondialdehyde) and liver damage without markedly decreasing hepatic lipid accumulation. WEC exerted similar effects although with decreased efficacy. In addition, WEC and phenethylamine decreased the methylglyoxal levels and increased the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels in the liver. Methylglyoxal is generated from substrates of GAPDH, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. These facts indicate that methylglyoxal triggers oxidation of accumulated lipid, which generates malondialdehyde and consequently induces liver damage. Suppression of generation of toxic aldehydes by WEC and phenethylamine was also confirmed by maintaining hepatic cysteine, highly reactive to aldehydes. Thus, trace amounts of phenethylamine alleviate HFD-induced liver damage by regulating methylglyoxal via increase of GAPDH. npj Science of Food (2021) 5:22 ; https://doi.org/10.1038/s41538-021-00105-3 1234567890():,; INTRODUCTION lipid deposition-induced oxidative stress in the liver contributes to Chlorella pyrenoidosa, a freshwater unicellular green alga, and its the pathogenesis of NAFLD13,14, whereas the liver contains potent water extract have a long history of usage as food supplements. antioxidant enzyme systems, such as the superoxide dismutase Various animal studies and clinical trials have reported that C. -

Protective Effects of Geniposide and Genipin Against Hepatic Ischemia/Reperfusion Injury in Mice
Original Article Biomol Ther 21(2), 132-137 (2013) Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice Joonki Kim1, Hyo-Yeon Kim2 and Sun-Mee Lee2,* 1Natural Medicine Center, Korea Institute of Science & Technology, Gangneung 210-340, 2School of Pharmacy, Sungkyunkwan University, Suwon 440-746, Republic of Korea Abstract Geniposide is an active product extracted from the gardenia fruit, and is one of the most widely used herbal preparations for liver disorders. This study examined the cytoprotective properties of geniposide and its metabolite, genipin, against hepatic ischemia/ reperfusion (I/R) injury. C57BL/6 mice were subjected to 60 min of ischemia followed by 6 h of reperfusion. Geniposide (100 mg/ kg) and genipin (50 mg/kg) were administered orally 30 min before ischemia. In the I/R mice, the levels of serum alanine amino- transferase and hepatic lipid peroxidation were elevated, whereas hepatic glutathione/glutathione disulfide ratio was decreased. These changes were attenuated by geniposide and genipin administration. On the other hand, increased hepatic heme oxygen- ase-1 protein expression was potentiated by geniposide and genipin administration. The increased levels of tBid, cytochrome c protein expression and caspase-3 activity were attenuated by geniposide and genipin. Increased apoptotic cells in the I/R mice were also significantly reduced by geniposide and genipin treatment. Our results suggest that geniposide and genipin offer signifi- cant hepatoprotection against I/R injury by reducing oxidative stress and apoptosis. Key Words: Geniposide, Genipin, Ischemia, Reperfusion, Liver, Apoptosis INTRODUCTION 2004). Oral administration of geniposide increased hepatic glutathione (GSH) content, which is responsible for hepatopro- Ischemia/reperfusion (I/R) injury to the liver is of clinical im- tection against aflatoxin B1-induced liver injury in rats (Kanget portance in humans after hemorrhagic and cardiogenic shock, al., 1997). -

GSTA4 (NM 001512) Human Tagged ORF Clone – RC202130 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC202130 GSTA4 (NM_001512) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: GSTA4 (NM_001512) Human Tagged ORF Clone Tag: Myc-DDK Symbol: GSTA4 Synonyms: GSTA4-4; GTA4 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC202130 ORF sequence Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGGCAGCAAGGCCCAAGCTCCACTATCCCAACGGAAGAGGCCGGATGGAGTCCGTGAGATGGGTTTTAG CTGCCGCCGGAGTCGAGTTTGATGAAGAATTTCTGGAAACAAAAGAACAGTTGTACAAGTTGCAGGATGG TAACCACCTGCTGTTCCAACAAGTGCCCATGGTTGAAATTGACGGGATGAAGTTGGTACAGACCCGAAGC ATTCTCCACTACATAGCAGACAAGCACAATCTCTTTGGCAAGAACCTCAAGGAGAGAACCCTGATTGACA TGTACGTGGAGGGGACACTGGATCTGCTGGAACTGCTTATCATGCATCCTTTCTTAAAACCAGATGATCA GCAAAAGGAAGTGGTTAACATGGCCCAGAAGGCTATAATTAGATACTTTCCTGTGTTTGAAAAGATTTTA AGGGGTCACGGACAAAGCTTTCTTGTTGGTAATCAGCTGAGCCTTGCAGATGTGATTTTACTCCAAACCA TTTTAGCTCTAGAAGAGAAAATTCCTAATATCCTGTCTGCATTTCCTTTCCTCCAGGAATACACAGTGAA ACTAAGTAATATCCCTACAATTAAGAGATTCCTTGAACCTGGCAGCAAGAAGAAGCCTCCCCCTGATGAA ATTTATGTGAGAACCGTCTACAACATCTTTAGGCCA ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT ACAAGGATGACGACGATAAGGTTTAA This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene -

Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders
Review Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders Giuseppina Barrera 1,*, Stefania Pizzimenti 1, Martina Daga 1, Chiara Dianzani 2, Alessia Arcaro 3, Giovanni Paolo Cetrangolo 3, Giulio Giordano 4, Marie Angele Cucci 1, Maria Graf 4 and Fabrizio Gentile 3 1 Dipartimento di Scienze Cliniche e Biologiche, Università di Torino, 10124 Turin, Italy; [email protected] (S.P.); [email protected] (M.D.); [email protected] (M.A.C.) 2 Dipartimento di Scienze e Tecnologia del Farmaco, Università di Torino, 10124 Turin, Italy; [email protected] 3 Dipartimento di Medicina e Scienze della Salute “V. Tiberio”, Università del Molise, 86100 Campobasso, Italy; [email protected] (A.A.); [email protected] (G.P.C.); [email protected] (F.G.) 4 Presidio Ospedaliero “A. Cardarelli”, Azienda Sanitaria Regione Molise, 86100 Campobasso, Italy; [email protected] (G.G.); [email protected] (M.G.) * Correspondence: [email protected] Received: 29 June 2018; Accepted: 27 July 2018; Published: 30 July 2018 Abstract: Among the various mechanisms involved in aging, it was proposed long ago that a prominent role is played by oxidative stress. A major way by which the latter can provoke structural damage to biological macromolecules, such as DNA, lipids, and proteins, is by fueling the peroxidation of membrane lipids, leading to the production of several reactive aldehydes. Lipid peroxidation-derived aldehydes can not only modify biological macromolecules, by forming covalent electrophilic addition products with them, but also act as second messengers of oxidative stress, having relatively extended lifespans. Their effects might be further enhanced with aging, as their concentrations in cells and biological fluids increase with age. -

276.Full.Pdf
[CANCER RESEARCH 40, 276-282, February 19801 0008-5472/80/0040-0000$02.00 Comparison of the Mutagenicities of Malondialdehyde and the Side Products Formed during Its Chemical Synthesis1 Lawrence J. Marnett2 and Melissa A. Tuttle Department of Chemistry, Wayne State University. Detroit, Michigan 48202 ABSTRACT (9, 15). It is believed that the formation of these polymers can be avoided by using very dilute solutions of TEP or TMP for the Malondialdehyde, a product of polyunsaturated fatty acid hydrolyses (15). Since MDA is a relatively weak mutagen, high metabolism and degradation, has been reported to be muta concentrations must be used to detect a mutagenic response genic and carcinogenic. The malondialdehyde used for testing (16, 21). This necessitates the use of concentrated solutions was generated by the acidic hydrolysis of tetraalkoxypropanes. of TEP or TMP for the preparation of the MDA solutions. We have studied the production of compounds mutagenic to MDA is a moderatelyweak acid (pK@,,4.46)which exists as Sa!mone!!a typhimurium strain his D 3052 following the hy its conjugate base at physiological pH (Ref. 17; Equation A). drolysis of tetraalkoxypropanes. The major mutagenic com pound produced from tetraethoxypropane is 18-ethoxy-acrolein 0 0 (90 to 100 revertants4tmol) and not malondialdehyde (3 to 5 II @ revertants/jsmol). Hydrolysis of tetramethoxypropane pro H )@ H pKa duces two compounds, $-methoxy-acrolein (125 to 160 re _________ H@ ,,C@ vertants4tmol) and 3,3-dimethoxypropionaldehyde (105 to II 135 revertants/@mol), which are more mutagenic than is ma ,C@ @ londialdehyde. Using standard conditions for the hydrolysis of H H tetraethoxypropane, the yield of malondialdehyde is 25%, and the yield of ,6-ethoxyacrolein is 13%. -

A Thesis Entitled Identification and Characterization of a Zebrafish
A Thesis Entitled Identification and Characterization of A Zebrafish Glutathione S-Transferase Pi By Maryam S Abunnaja Submitted to the Graduate Faculty as a Partial Fulfillment of the Requirement for the Master of Science Degree in Pharmacology and Toxicology Dr. Ming-Cheh Liu, Committee Chair Dr. Ezdihar Hassoun, Committee Member Dr. Zahoor Shah, Committee Member Dr. Patricia Komuniecki, Dean College Graduate Studies The University of Toledo May 2013 Copyright 2013, Maryam S Abunnaja This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Identification and Characterization of A Zebrafish Glutathione S-Transferase Pi By Maryam S Abunnaja Submitted to the Graduate Faculty as a Partial Fulfillment of the Requirement for the Master of Science Degree in Pharmacology and Toxicology May 2013 Glutathione S-transferases (GSTs) are a major group of Phase II enzymes involved in the detoxification of certain endogenous compounds such as reactive oxygen species (ROS) generated during metabolism, as well as in the detoxification of xenobiotics including drugs. In recent years, the zebrafish has increasingly been used as a promising animal model for biomedical research. This study is part of an effort to establish the zebrafish as a model for studying GST-mediated glutathione conjugation of xenobiotics. By searching the GenBank database, we have identified sequences encoding fifteen zebrafish GSTs. Using a computer algorithm available at the Genebee website, a dendrogram of the fifteen zebrafish GSTs was generated. A zebrafish GST Pi was subsequently cloned, expressed, and purified. An enzymatic assay for purified GST Pi was established using 1- chloro-2, 4-dinitrobenzene (CDNB) as a substrate. -

Glutathione S-Transferase Enzyme
Haematologica 2000; 85:573-579 Hematopoiesis & Growth Factors original paper Glutathione S-transferase enzyme expression in hematopoietic cell lines implies a differential protective role for T1 and A1 isoenzymes in erythroid and for M1 in lymphoid lineages LIHUI WANG, MICHAEL J. GROVES, MARY D. HEPBURN, DAVID T. BOWEN Department of Molecular and Cellular Pathology, University of Dundee Medical School, Dundee, UK ABSTRACT Background and Objectives. Glutathione S-trans- exposure to xenobiotics that are substrates for ferases (GSTs) are phase II metabolizing enzymes these enzymes. which catalyze the conjugation of glutathione (GSH) ©2000, Ferrata Storti Foundation to electrophilic substrates and possess selenium- independent glutathione peroxidase activity. The Key words: glutathione S-transferase, genotoxicity, GST enzyme family includes the cytosolic isoforms antioxidant, hematopoietic cell lines GST-α (GSTA), µ (GSTM), π (GSTP), θ (GSTT) and σ (GSTS). GSTT1, P1 and M1 are polymorphic and altered polymorphic frequency of genes encoding igher organisms have evolved complex mech- these proteins has been suggested as a potential anisms by which they can protect themselves risk factor for the development of hematopoietic from environmental challenge. The metabo- malignancies. Overexpression of GSTs has also H lism and detoxification of xenobiotic toxins provides been implicated in chemotherapeutic drug resis- tance. This study was undertaken to elucidate the the first line of intracellular defence and this is medi- potential functional relevance of these genetic poly- ated by enzyme superfamilies, including the cyto- morphisms in hematopoiesis. chrome p450, glutathione S-transferase (GST) and N-acetyltransferases. Glutathione S-transferase Design and Methods. GST genotype of 14 genes encode for five families of cytosolic enzymes: hematopoietic cell lines was determined by poly- GSTs-α (GSTA), µ (GSTM), π (GSTP), θ (GSTT) and merase-chain-reaction (PCR). -

GSTA4 Purified Maxpab Mouse Polyclonal Antibody (B01P)
GSTA4 purified MaxPab mouse mammalian glutathione S-transferases have been polyclonal antibody (B01P) identified: alpha, kappa, mu, omega, pi, sigma, theta and zeta. This gene encodes a glutathione S-tranferase Catalog Number: H00002941-B01P belonging to the alpha class. The alpha class genes, which are located in a cluster on chromosome 6, are Regulatory Status: For research use only (RUO) highly related and encode enzymes with glutathione peroxidase activity that function in the detoxification of Product Description: Mouse polyclonal antibody raised lipid peroxidation products. Reactive electrophiles against a full-length human GSTA4 protein. produced by oxidative metabolism have been linked to a number of degenerative diseases including Parkinson's Immunogen: GSTA4 (AAH15523, 1 a.a. ~ 222 a.a) disease, Alzheimer's disease, cataract formation, and full-length human protein. atherosclerosis. [provided by RefSeq] Sequence: References: MAARPKLHYPNGRGRMESVRWVLAAAGVEFDEEFLE 1. Glutathione S-transferase alpha 4 induction by TKEQLYKLQDGNHLLFQQVPMVEIDGMKLVQTRSILHY activator protein 1 in colorectal cancer. Yang Y, Huycke IADKHNLFGKNLKERTLIDMYVEGTLDLLELLIMHPFLK MM, Herman TS, Wang X. Oncogene. 2016 Apr 11. PDDQQKEVVNMAQKAIIRYFPVFEKILRGHGQSFLVGN [Epub ahead of print] QLSLADVILLQTILALEEKIPNILSAFPFLQEYTVKLSNIP TIKRFLEPGSKKKPPPDEIYVRTVYNIFRP Host: Mouse Reactivity: Human Applications: WB-Ce, WB-Tr (See our web site product page for detailed applications information) Protocols: See our web site at http://www.abnova.com/support/protocols.asp or product page for detailed protocols Storage Buffer: In 1x PBS, pH 7.4 Storage Instruction: Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Entrez GeneID: 2941 Gene Symbol: GSTA4 Gene Alias: DKFZp686D21185, GSTA4-4, GTA4 Gene Summary: Cytosolic and membrane-bound forms of glutathione S-transferase are encoded by two distinct supergene families. -
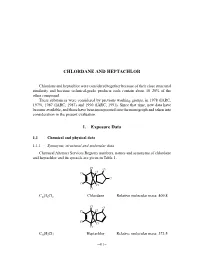
Chlordane and Heptachlor
CHLORDANE AND HEPTACHLOR Chlordane and heptachlor were considered together because of their close structural similarity and because technical-grade products each contain about 10–20% of the other compound. These substances were considered by previous working groups, in 1978 (IARC, 1979), 1987 (IARC, 1987) and 1990 (IARC, 1991). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Chemical Abstract Services Registry numbers, names and synonyms of chlordane and heptachlor and its epoxide are given in Table 1. Cl Cl Cl Cl Cl Cl Cl Cl C10H6Cl8 Chlordane Relative molecular mass: 409.8 Cl Cl Cl Cl Cl Cl Cl C10H5Cl7 Heptachlor Relative molecular mass: 373.5 –411– 412 IARC MONOGRAPHS VOLUME 79 Table 1. Chemical Abstract Services Registry numbers, names and synonyms of chlordane, heptachlor and its epoxide Name CAS Reg. Nosa Chem. Abstr. namesb and synonyms Chlordane 57-74-9 ENT 9932; 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a- (39400-80-1); hexahydro-4,7-methano-1H-indene; 1,2,4,5,6,7,8,8- 53637-13-1) octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano- indene (IUPAC); octachloro-4,7-methanotetrahydro- indane; 1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetra- hydro-4,7-methanoindan; OMS 1437 Technical-grade 12789-03-6 chlordane cis-Chlordane 5103-71-9 α-Chlordan; α-chlordane; cis-chlordan; (152322-29-7; (1α,2α,3aα,4β,7β,7aα)-1,2,4,5,6,7,8,8-octachloro- 22212-52-8;