Family Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocps) Used in the NM Family Planning Program (FPP
The following slides are intended to familiarize nurses and clinicians with oral contraceptive pills (OCPs) used in the NM Family Planning Program (FPP). The FPP does not intend for this information to supersede the Family Planning Protocol particularly on the requirement for Public Health Nurses in the Public Health Offices to consult a clinician as stated in the Protocol when in doubt or if it is necessary to switch the client’s OCP type. 1 2 Combined oral contraceptives (COCs) contain two hormones; estrogen and progestin. In general, any combined OCP is good for most women who are eligible to take estrogen according to the CDC U.S. Medical Eligibility Criteria (MEC). Once again, refer to the US MEC chart to find out if OCP is a suitable choice for clients with specific health conditions. To learn a little bit about what each hormone does, the FPP is providing the following summary: Estrogen: provides endometrial stability = menstrual cycle control. A higher estrogen dose increases the venous thromboembolism (VTE) or clot risk but OCP clot risk is still less harmful than the clot risk related to pregnancy and giving birth. Progestin: provides most of the contraceptive effect by ‐Preventing luteinizing hormone (LH) surge /ovulation ‐Thickening the cervical mucus to prevent sperm entry. Two major OCP formations are available. Monophasic: There is only one dose of estrogen and progestin in each active pill in the packet; and Multiphasic: There are varying doses of hormones, particularly progestin in the active pills. 3 Section 3 of the FPP Protocol contains the OCP Substitute Table, which groups OCPs into 6 classes according to the estrogen dosage, the type of progestin and the formulations. -

UNITED STATES PATENT OFFICE 2,636,042 WATER-SOLUBLE HORMONE COMPOUNDS Ralph Salkin, Jackson Heights, N.Y., Assignor to S
Patented Apr. 21, 1953 2,636,042 UNITED STATES PATENT OFFICE 2,636,042 WATER-SOLUBLE HORMONE COMPOUNDS Ralph Salkin, Jackson Heights, N.Y., assignor to S. B. Penick and Company, New York, N. Y., a corporation of Delaware No Drawing. Application July 8, 1949 Serial No. 103,759 5 Claims. (C. 260-39.4) 1. 2 My invention relates to an improvement in the ether, and the sulfate is then Salted out of the manufacture of water-soluble compounds of the aqueous solution by the addition of a, caustic estrane series, and in particular it is concerned solution under cooling. The liberated hormone With an improvement in the synthesis of alkali sulfate is extracted into a suitable Solvent, for and alkaline-earth metal salts of the sulfates of 5 instance butanol, pyridine being preferred how the estranes. ever. The hormone sulfate solution is exhaus The estranes to which my invention applies are tively extracted with ether to remove the solvent. steroids having a free hydroxyl group in the The resultant semicrystalline product is recrys 3-position and a hydroxy or keto group in the tallized from a dilute monohydric alcohol. Or 17-position of the molecule, such as estrone, O Water to give the pure sterol.ester. equilin, equilenin, estradiol and similar com In order to get pure ester Salts, I have found pounds. it essential that the tertiary amine-sulfur trioxide These products which are commonly known as adduct be absolutely pure when being reacted With conjugated estrogens can be obtained from nat the hormones. Improved yields and more readily ural sources such as the urine of pregnant mares purifiable light colored granular products result, or of stallions. -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Pp375-430-Annex 1.Qxd
ANNEX 1 CHEMICAL AND PHYSICAL DATA ON COMPOUNDS USED IN COMBINED ESTROGEN–PROGESTOGEN CONTRACEPTIVES AND HORMONAL MENOPAUSAL THERAPY Annex 1 describes the chemical and physical data, technical products, trends in produc- tion by region and uses of estrogens and progestogens in combined estrogen–progestogen contraceptives and hormonal menopausal therapy. Estrogens and progestogens are listed separately in alphabetical order. Trade names for these compounds alone and in combination are given in Annexes 2–4. Sales are listed according to the regions designated by WHO. These are: Africa: Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, South Africa, Swaziland, Togo, Uganda, United Republic of Tanzania, Zambia and Zimbabwe America (North): Canada, Central America (Antigua and Barbuda, Bahamas, Barbados, Belize, Costa Rica, Cuba, Dominica, El Salvador, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago), United States of America America (South): Argentina, Bolivia, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guyana, Paraguay, -

BRS Pharmacology
Pharmacology Gary C. Rosenfeld, Ph.D. Professor Department of Integrated Biology and Pharmacology and Graduate School of Biomedical Sciences Assistant Dean for Education Programs University of Texas Medical School at Houston Houston, Texas David S. Loose, Ph.D. Associate Professor Department of Integrated Biology and Pharmacology and Graduate School of Biomedical Sciences University of Texas Medical School at Houston Houston, Texas With special contributions by Medina Kushen, M.D. William Beaumont Hospital Royal Oak, Michigan Todd A. Swanson, M.D., Ph.D. William Beaumont Hospital Royal Oak, Michigan Acquisitions Editor: Charles W. Mitchell Product Manager: Stacey L. Sebring Marketing Manager: Jennifer Kuklinski Production Editor: Paula Williams Copyright C 2010 Lippincott Williams & Wilkins 351 West Camden Street Baltimore, Maryland 21201-2436 USA 530 Walnut Street Philadelphia, PA 19106 All rights reserved. This book is protected by copyright. No part of this book may be reproduced in any form or by any means, including photocopying, or utilized by any information storage and retrieval system without written permission from the copyright owner. The publisher is not responsible (as a matter of product liability, negligence or otherwise) for any injury resulting from any material contained herein. This publication contains information relating to general principles of medical care which should not be construed as specific instructions for individual patients. Manufacturers’ product information and package inserts should be reviewed for current information, including contraindications, dosages and precautions. Printed in the United States of America Library of Congress Cataloging-in-Publication Data Rosenfeld, Gary C. Pharmacology / Gary C. Rosenfeld, David S. Loose ; with special contributions by Medina Kushen, Todd A. -

Norgestrel and Gestodene Stimulate Breast Cancer Cell Growth Through an Oestrogen Receptor Mediated Mechanism
Br. J. Cancer (1993), 67, 945-952 '." Macmillan Press Ltd., 1993 Br. J. Cancer (1993), 67, 945-952 1993 Norgestrel and gestodene stimulate breast cancer cell growth through an oestrogen receptor mediated mechanism W.H. Catherino, M.H. Jeng & V.C. Jordan Department ofHuman Oncology, University of Wisconsin Comprehensive Cancer Center, 600 Highland Avenue, Madison, Wisconsin 53792, USA. Summary There is great concern over the long-term influence of oral contraceptives on the development of breast cancer in women. Oestrogens are known to stimulate the growth of human breast cancer cells, and this laboratory has previously reported (Jeng & Jordan, 1991) that the 19-norprogestin norethindrone could stimulate the proliferation of MCF-7 human breast cancer cells. We studied the influence of the 19-norprogestins norgestrel and gestodene compared to a 'non' 19- norprogestin medroxyprogesterone acetate (MPA) on MCF-7 cell proliferation. The 19-norprogestins stimulated proliferation at a concentration of 10-8 M, while MPA could not stimulate proliferation at concentrations as great as 3 x 10-6 M. The stimulatory activity of the 19-norprogestins could be blocked by the antioestrogen ICI 164,384, but not by the antiprogestin RU486. Transfection studies with the reporter plasmids containing an oestrogen response element or progesterone response element (vitERE-CAT, pS2ERE-CAT, and PRE15-CAT) were performed to determine the intracel- lular action of norgestrel and gestodene. The 19-norprogestins stimulated the vitERE-CAT activity maximally at 10-6 M, and this stimulation was inhibited by the addition of ICI 164,384. MPA did not stimulate vitERE-CAT activity. A single base pair alteration in the palindromic sequence of vitERE (resulting in the pS2ERE) led to a dramatic decrease in CAT expression by the 19-norprogestins, suggesting that the progestin activity required specific response element base sequencing. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -
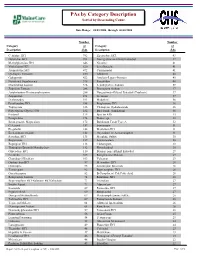
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Labeling and Synthesis of Estrogens and Their Metabolites
Labeling and Synthesis of Estrogens and Their Metabolites Paula Kiuru University of Helsinki Faculty of Science Department of Chemistry Laboratory of Organic Chemistry P.O. Box 55, 00014 University of Helsinki, Finland ACADEMIC DISSERTATION To be presented with the permission of the Faculty of Science of the University of Helsinki, for public criticism in Auditorium A110 of the Department of Chemistry, A. I. Virtasen Aukio 1, Helsinki, on June 18th, 2005 at 12 o'clock noon Helsinki 2005 ISBN 952-91-8812-9 (paperback) ISBN 952-10-2507-7 (PDF) Helsinki 2005 Valopaino Oy. 1 ABSTRACT 3 ACKNOWLEDGMENTS 4 LIST OF ORIGINAL PUBLICATIONS 5 LIST OF ABBREVIATIONS 6 1. INTRODUCTION 7 1.1 Nomenclature of estrogens 8 1.2 Estrogen biosynthesis 10 1.3 Estrogen metabolism and cancer 10 1.3.1 Estrogen metabolism 11 1.3.2 Ratio of 2-hydroxylation and 16α-hydroxylation 12 1.3.3 4-Hydroxyestrogens and cancer 12 1.3.4 2-Methoxyestradiol 13 1.4 Structural and quantitative analysis of estrogens 13 1.4.1 Structural elucidation 13 1.4.2 Analytical techniques 15 1.4.2.1 GC/MS 16 1.4.2.2 LC/MS 17 1.4.2.3 Immunoassays 18 1.4.3 Deuterium labeled internal standards for GC/MS and LC/MS 19 1.4.4 Isotopic purity 20 1.5 Labeling of estrogens with isotopes of hydrogen 20 1.5.1 Deuterium-labeling 21 1.5.1.1 Mineral acid catalysts 21 1.5.1.2 CF3COOD as deuterating reagent 22 1.5.1.3 Base-catalyzed deuterations 24 1.5.1.4 Transition metal-catalyzed deuterations 25 1.5.1.5 Deuteration without catalyst 27 1.5.1.6 Halogen-deuterium exchange 27 1.5.1.7 Multistep labelings 28 1.5.1.8 Summary of deuterations 30 1.5.2 Enhancement of deuteration 30 1.5.2.1 Microwave irradiation 30 1.5.2.2 Ultrasound 31 1.5.3 Tritium labeling 32 1.6 Deuteration estrogen fatty acid esters 34 1.7 Synthesis of 2-methoxyestradiol 35 1.7.1 Halogenation 35 1.7.2 Nitration of estrogens 37 1.7.3 Formylation 38 1.7.4 Fries rearrangement 39 1.7.5 Other syntheses of 2-methoxyestradiol 39 1.7.6 Synthesis of 4-methoxyestrone 40 1.8 Synthesis of 2- and 4-hydroxyestrogens 41 2. -

REVIEW Steroid Sulfatase Inhibitors for Estrogen
99 REVIEW Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers Atul Purohit and Paul A Foster1 Oncology Drug Discovery Group, Section of Investigative Medicine, Imperial College London, Hammersmith Hospital, London W12 0NN, UK 1School of Clinical and Experimental Medicine, Centre for Endocrinology, Diabetes and Metabolism, University of Birmingham, Birmingham B15 2TT, UK (Correspondence should be addressed to P A Foster; Email: [email protected]) Abstract Estrogens and androgens are instrumental in the maturation of in vivo and where we currently stand in regards to clinical trials many hormone-dependent cancers. Consequently,the enzymes for these drugs. STS inhibitors are likely to play an important involved in their synthesis are cancer therapy targets. One such future role in the treatment of hormone-dependent cancers. enzyme, steroid sulfatase (STS), hydrolyses estrone sulfate, Novel in vivo models have been developed that allow pre-clinical and dehydroepiandrosterone sulfate to estrone and dehydroe- testing of inhibitors and the identification of lead clinical piandrosterone respectively. These are the precursors to the candidates. Phase I/II clinical trials in postmenopausal women formation of biologically active estradiol and androstenediol. with breast cancer have been completed and other trials in This review focuses on three aspects of STS inhibitors: patients with hormone-dependent prostate and endometrial 1) chemical development, 2) biological activity, and 3) clinical cancer are currently active. Potent STS inhibitors should trials. The aim is to discuss the importance of estrogens and become therapeutically valuable in hormone-dependent androgens in many cancers, the developmental history of STS cancers and other non-oncological conditions. -

University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN
This dissertation has been Mic 61-2820 microfilmed exactly as received BESCH, Paige Keith. ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG. The Ohio State University, Ph.D., 1961 Chemistry, biological University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Paige Keith Besch, B. S., M. S. The Ohio State University 1961 Approved by Katharine A. Brownell Department of Physiology DEDICATION This work is dedicated to my wife, Dr. Norma F. Besch. After having completed her graduate training, she was once again subjected to almost social isolation by the number of hours I spent away from home. It is with sincerest appreciation for her continual encouragement that I dedi cate this to her. ACKNOWLEDGMENTS I wish to acknowledge the assistance and encourage ment of my Professor, Doctor Katharine A. Brownell. Equally important to the development of this project are the experience and information obtained through the association with Doctor Frank A. Hartman, who over the years has, along with Doctor Brownell, devoted his life to the development of many of the techniques used in this study. It is also with extreme sincerity that I wish to ac knowledge the assistance of Mr. David J. Watson. He has never complained when asked to work long hours at night or weekends. Our association has been a fruitful one. I also wish to acknowledge the encouragement of my former Professor, employer and good friend, Doctor Joseph W. -

Oral Contraceptives
ORAL CONTRACEPTIVES STRENGTH DRUG ESTROGEN PROGESTIN (ESTROGEN/PROGESTIN) MONOPHASIC Aviane 28, Lessina, Lutera, Orsythia, Ethinyl estradiol Levonorgestrel 20mcg/0.1mg Sronyx† Beyaz*, YAZ Ethinyl estradiol Drospirenone 20mcg/3mg [Gianvi, Loryna, Vestura]† Brevicon, Modicon Ethinyl estradiol Norethindrone 35mcg/0.5mg [Necon 0.5/35, Nortrel 0.5/35]† Desogen Ethinyl estradiol Desogestrel 30mcg/0.15mg [Apri, Emoquette, Reclipsen, Solia]† Femcon Fe, Ovcon 35 Ethinyl estradiol Norethindrone 35mcg/0.4mg [Balziva, Briellyn, Philith, Vyfemla Zenchent, Zenchent Fe]† Loestrin 21 1/20, Loestrin Fe 1/20, Ethinyl estradiol Norethindrone acetate 20mcg/1mg Loestrin 24 Fe, Minastrin 24 Fe [Gildess Fe 1/20, Junel 1/20, Junel Fe 1/20, Larin 1/20, Larin Fe 1/20, Microgestin 1/20, Microgestin Fe 1/20]† Loestrin 21 1.5/30, Loestrin Fe 1.5/30 Ethinyl estradiol Norethindrone acetate 30mcg/1.5mg [Gildess Fe 1.5/30, Junel 1.5/30, Junel Fe 1.5/30, Microgestin 1.5/30, Microgestin Fe 1.5/30]† Lo/Ovral Ethinyl estradiol Norgestrel 30mcg/0.3mg [Cryselle, Low-Ogestrel]† Lybrel Ethinyl estradiol Levonorgestrel 20mcg/0.09mg [Amethyst]† [Altavera, Levora, Marlissa, Portia]† Ethinyl estradiol Levonorgestrel 30mcg/0.15mg Norinyl 1/35, Ortho-Novum 1/35 Ethinyl estradiol Norethindrone 35mcg/1mg [Alyacen 1/35, Cyclafem 1/35, Dasetta 1/35, Necon 1/35, Nortrel 1/35]† Necon 1/50, Norinyl 1/50 Mestranol Norethindrone 50mcg/1mg Ogestrel 0.5/50† Ethinyl estradiol Norgestrel 50mcg/0.5mg Ortho-Cyclen Ethinyl estradiol Norgestimate 35mcg/0.25mg [MonoNessa, Previfem, Sprintec]† Ovcon