Chapter 8. Plankton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

St. Lucie and Indian River Counties Water Resources Study
St. Lucie and Indian River Counties Water Resources Study Final Summary Report November 2009 Prepared for: South Florida Water Management District St. Johns River Water Management District St Lucie and Indian River Counties Water Resource Study St Lucie and Indian River Counties Water Resources Study Executive Summary Study Purpose The purpose of this study was to evaluate the potential for capturing excess water that is currently being discharged to the Indian River Lagoon in northern St. Lucie County and southern Indian River County and making it available for beneficial uses. The study also evaluated the reconnection of the C-25 Basin in the South Florida Water Management District (SFWMD) and C-52 in the St. Johns River Water Management District (SJRWMD) so that available water supplies could be conveyed to meet demands across jurisdictional boundaries. The study objectives were to: Identify the quantity and timing of water available for diversion and storage; Identify water quality information needed to size water quality improvement facilities; Identify and provide cost estimates for the improvements and modifications to the existing conveyance systems necessary for excess runoff diversion and storage; Identify, develop cost estimates, and evaluate conceptual alternatives for storing excess runoff, and Provide conceptual designs and cost estimates for the highest ranked alternative in support of feasibility analysis and a future Basis of Design Report. Study Process The study process consisted of the following activities: Data compilation and analysis, Identification of alternative plans, Evaluation of alternative plans, Identification of the preferred plan, and Development of an implementation strategy. St Lucie and Indian River Counties Water Resource Study Formal stakeholder meetings were conducted throughout the study. -

Water Resources Brevard County, Florida
STATE OF FLORIDA STATE BOARD OF CONSERVATION DIVISION OF GEOLOGY FLORIDA GEOLOGICAL SURVEY Robert O. Vernon, Director REPORT OF INVESTIGATIONS NO. 28 WATER RESOURCES OF BREVARD COUNTY, FLORIDA By D. W. Brown, W. E. Kenner, J. W. Crooks, and J. B. Foster U. S. Geological Survey Prepared by the UNITED STATES GEOLOGICAL SURVEY in cooperation with the CENTRAL AND SOUTHERN FLORIDA FLOOD CONTROL DISTRICT the U. S. ARMY, CORPS OF ENGINEERS and the. FLORIDA GEOLOGICAL SURVEY TALLAHASSEE 1962 /AJ.z7s FLORIDA STATE BOA ~"• OF CONSERVATION FARRIS BRYANT Governor TOM ADAMS J. EDWIN LARSON Secretary of State Treasurer THOMAS D. BAILEY RICHARD ERVIN Stperintendent of Public Instruction Attorney General RAY E. GREEN DOYLE CONNER Comptroller Commissioner of Agriculture W. RANDOLPH HODGES Director . ii LETTER OF TRANSMITTAL Qfo'ida Ge)oloqicaf 5 urvej January 11, 1962 Honorable Farris Bryant, Chairman Florida State Board of Conservation Tallahassee, Florida Dear Governor Bryant: The Florida Geological Survey is pleased to publish as Report of In- vestigations No. 28, a comprehensive study of the water resources of Brevard County. This report was prepared by Messrs. D. W. Brown, W. E. Kenner, J. W. Crooks, and J. B. Foster, of the U. S. Geological Survey, in cooperation with the Central and Southern Florida Flood Control District; U. S. Army, Corps of Engineers; and the Florida Geological Survey. This is a very timely study, since the development of adequate supplies of fresh water and the prevention and alleviation of flooding are the principal water problems in Brevard County. The rapid expansion of popu- lation and the development of new industries associated with the space effort have made large demands for increased supplies of fresh water, particularly in the Atlantic Coastal Ridge area on Merritt Island and in the barrier beach area. -

W. MICHAEL DENNIS, Ph.D
W. MICHAEL DENNIS, Ph.D. Areas of Specialization: Wetland delineation, permitting and mitigation; plant taxonomy and ecology; remote sensing and aerial photointerpretation; threatened and endangered (T&E) species; and wildlife evaluations. Experience: President, Breedlove, Dennis & Associates, Inc. (BDA), Winter Park, Florida. 1997 to present. Principal, BDA, Winter Park, Florida. 1984 to present. Vice President, BDA, Winter Park, Florida. 1983 to 1997. Senior Scientist, Breedlove & Associates, Inc., Gainesville, Florida. 1981 to 1983. Projects and responsibilities included development of technical data and management of projects in the following areas: Vegetation analysis and wetlands jurisdictional evaluations for land development activities in Alachua, Baker, Bay, Brevard, Broward, Charlotte, Citrus, Clay, Collier, Columbia, Dade, Dixie, Duval, Escambia, Flagler, Franklin, Gadsden, Gilchrist, Hamilton, Hardee, Hendry, Hernando, Highlands, Hillsborough ,Indian River, Jackson, Lake, Lee, Leon, Levy, Liberty, Manatee, Marion, Martin, Monroe, Nassau, Orange, Osceola, Palm Beach, Pasco, Pinellas, Polk, Putnam, Santa Rosa, Sarasota, Seminole, St. Johns, St. Lucie, Sumter, Suwannee, Taylor, Volusia, Wakulla, Walton, and Washington counties in Florida. Vegetation mapping of plant communities in Florida, Georgia, South Carolina, Alabama, Tennessee, Virginia, Kentucky, New Jersey, Mississippi, and North Carolina. Wetlands evaluations for phosphate, sand, and limerock mining activities. Wetland evaluations and permitting for major theme parks -

St. Johns River Water Supply Impact Study (WSIS)
St. Johns River Water Supply Impact Study (WSIS) Michael G. Cullum, P.E. Chief, Bureau of Engineering & Hydro Science St. Johns River Water Management District The Water Supply Impact study is the most comprehensive and rigorous investigation of the St. Johns River ever conducted. Major Conclusions • The St. Johns River can be used as an alternative water supply source with no more than negligible or minor effects. • Future land use changes, completion of the Upper St. Johns River Basin Project, and sea level rise reduce the effects of water withdrawals. • Potential for environmental effects varies along the river’s length. • The study provides peer-reviewed tools for use by the District and others. National Academy of Sciences National Research Council (NRC) Peer Review • Three-year process working with the NRC peer review committee. • Committee consisted of nine experts. • Six multi-day meetings, field trips and numerous teleconferences. • NRC ̶ 105 page report, December 2011 NRC Concluding Comment “The overall strategy of the study and the way it was implemented were appropriate and adequate to address the goals that the District established for the WSIS.” The first step: - Understand hydrology and hydraulics and predict the changes - Resulting from potential water withdrawals. • Watershed hydrology models predict inflows into the river. • River hydrodynamic model predicts river flow, level, and salinity. Baseline Scenario • 1995 Landuse • Water Supply Planning Base Year • Good Data set 1995-2006 • Stable USJ Project Conditions • Use for Calibration of Models Forecast Scenarios • 2030 Land-Use • Complete Upper SJR Projects • Fellsmere, • C1- Sawgrass Lakes • Three Forks Marsh • Conservative Sea Level Rise (14 cm) • Withdrawal Scenarios - 77.5 mgd, 155 mgd, & 262 mgd Watershed Models • Hydrologic Simulation Program – Fortran (HSPF) – 90 separate models – 11 in-house modelers – External Peer Review • Model for Upper SJR Basin • 55 mgd - near Lake Poinsett HSPF Modeling LULCDEMSoils D.E.M.Land CoverSoils Land-use, reaches, and rainfall gauges Uppert1 St. -

Assessment of Cyanotoxins in Florida's Lakes, Reservoirs And
Assessment of Cyanotoxins in Florida’s Lakes, Reservoirs and Rivers by Christopher D. Williams BCI Engineers and Scientists, Inc. Lakeland, FL. John W. Burns Andrew D. Chapman Leeanne Flewelling St. Johns River Water Management District Palatka, FL. Marek Pawlowicz Florida Department of Health/Bureau of Laboratories Jacksonville, FL. Wayne Carmichael Wright State University Dayton, OH. 2001 Executive Summary EXECUTIVE SUMMARY Harmful algal blooms (HABs) are population increases of algae above normal background levels and are defined by their negative impacts on the environment, the economy, and human health. Historically, many of Florida's largest and most utilized freshwater and estuarine systems have been plagued by occasional blooms of harmful algae. During the last decade, however, the frequency, duration, and concentration levels of these blooms in freshwater and brackish water have increased significantly, primarily due to changes in land utilization, changes in hydrology, increases in nutrient runoff, loss of aquatic vegetation, and a climate that is very conducive to algal growth and proliferation. In 1998, the Florida Harmful Algal Bloom Task Force was established to determine the extent to which HABs pose a problem for the state of Florida. Blue-green algae (cyanobacteria) were identified as top research priorities due to their potential to produce toxic chemicals and contaminate natural water systems. In June 1999, the St. Johns River Water Management District (SJRWMD) initiated a collaborative study in conjunction with the Florida Marine Research Institute, the Florida Department of Health, and Wright State University to determine the geographical distribution of various types of toxin-producing blue-green algae in Florida's surface waters and to positively identify any algal toxins present in these waters. -
St. Johns River Blueway by Dean Campbell River Overview
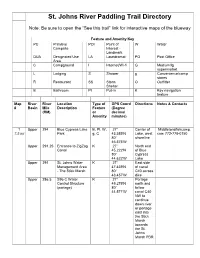
St. Johns River Paddling Trail Directory Note: Be sure to open the “See this trail” link for interactive maps of the blueway Feature and Amenity Key PC Primitive POI Point of W Water Campsite Interest - Landmark DUA Designated Use LA Laundromat PO Post Office Area C Campground I Internet/Wi-fi G Medium/lg supermarket L Lodging S Shower g Convenience/camp stores R Restaurant SS Storm O Outfitter Shelter B Bathroom PI Put-in K Key navigation feature Map River River Location Type of GPS Coord Directions Notes & Contacts # Basin Mile Description Feature (Degree (RM) or decimal Amenity minutes) 1 Upper 294 Blue Cypress Lake B, PI, W, 27° Center of Middletonsfishcamp. 7.5 mi Park g, C 43.589'N Lake, west com 772-778-0150 80° shoreline 46.575'W Upper 291.25 Entrance to ZigZag K 27° North end Canal 45.222'N of Blue 80° Cypress 44.622'W Lake Upper 291 St. Johns Water K 27° East side Management Area 47.439'N of canal - The Stick Marsh 80° C40 across 43.457'W dike Upper 286.5 S96 C Water K 27° Portage Control Structure 49.279'N north and (portage) 80° follow 44.571'W canal C40 NW to continue down river or portage east into the Stick Marsh towards the St. Johns Marsh PBR Upper 286.5 St. Johns Marsh – B, PI, W 27° East side Barney Green 49.393'N of canal PBR* 80° C40 across 42.537'W dike 2 Upper 286.5 St. Johns Marsh – B, PI, W 27° East side 22 mi Barney Green 49.393'N of canal *2 PBR* 80° C40 across day 42.537'W dike trip Upper 279.5 Great Egret PC 27° East shore Campsite 54.627'N of canal 80° C40 46.177'W Upper 277 Canal Plug in C40 K 27° In canal -

Floods in Florida Magnitude and Frequency
UNITED STATES EPARTMENT OF THE INTERIOR- ., / GEOLOGICAL SURVEY FLOODS IN FLORIDA MAGNITUDE AND FREQUENCY By R.W. Pride Prepared in cooperation with Florida State Road Department Open-file report 1958 MAR 2 CONTENTS Page Introduction. ........................................... 1 Acknowledgements ....................................... 1 Description of the area ..................................... 1 Topography ......................................... 2 Coastal Lowlands ..................................... 2 Central Highlands ..................................... 2 Tallahassee Hills ..................................... 2 Marianna Lowlands .................................... 2 Western Highlands. .................................... 3 Drainage basins ....................................... 3 St. Marys River. ......_.............................. 3 St. Johns River ...................................... 3 Lake Okeechobee and the everglades. ............................ 3 Peace River ....................................... 3 Withlacoochee River. ................................... 3 Suwannee River ...................................... 3 Ochlockonee River. .................................... 5 Apalachicola River .................................... 5 Choctawhatchee, Yellow, Blackwater, Escambia, and Perdido Rivers. ............. 5 Climate. .......................................... 5 Flood records ......................................... 6 Method of flood-frequency analysis ................................. 9 Flood frequency at a gaging -

Florida Fish and Wildlife Conservation Commission Statewide Alligator Harvest Data Summary
FWC Home : Wildlife & Habitats : Managed Species : Alligator Management Program FLORIDA FISH AND WILDLIFE CONSERVATION COMMISSION STATEWIDE ALLIGATOR HARVEST DATA SUMMARY YEAR AVERAGE LENGTH TOTAL HARVEST FEET INCHES 2000 8 8 2,552 2001 8 8.2 2,268 2002 8 3.7 2,164 2003 8 4.6 2,830 2004 8 5.8 3,237 2005 8 4.9 3,436 2006 8 4.8 6,430 2007 8 6.7 5,942 2008 8 5.1 6,204 2009 8 0 7,844 2010 7 10.9 7,654 2011 8 1.2 8,103 Provisional data 2000 STATEWIDE ALLIGATOR HARVEST DATA SUMMARY AVERAGE LENGTH TOTAL AREA NO AREA NAME FEET INCHES HARVEST 101 LAKE PIERCE 7 9.8 12 102 LAKE MARIAN 9 9.3 30 104 LAKE HATCHINEHA 8 7.9 36 105 KISSIMMEE RIVER (POOL A) 7 6.7 17 106 KISSIMMEE RIVER (POOL C) 8 8.3 17 109 LAKE ISTOKPOGA 8 0.5 116 110 LAKE KISSIMMEE 7 11.5 172 112 TENEROC FMA 8 6.0 1 402 EVERGLADES WMA (WCAs 2A & 2B) 8 8.2 12 404 EVERGLADES WMA (WCAs 3A & 3B) 8 10.4 63 405 HOLEY LAND WMA 9 11.0 2 500 BLUE CYPRESS LAKE 8 5.6 31 501 ST. JOHNS RIVER 1 8 2.2 69 502 ST. JOHNS RIVER 2 8 0.7 152 504 ST. JOHNS RIVER 4 8 3.6 83 505 LAKE HARNEY 7 8.7 65 506 ST. JOHNS RIVER 5 9 2.2 38 508 CRESCENT LAKE 8 9.9 23 510 LAKE JESUP 9 9.5 28 518 LAKE ROUSSEAU 7 9.3 32 520 LAKE TOHOPEKALIGA 9 7.1 47 547 GUANA RIVER WMA 9 4.6 5 548 OCALA WMA 9 8.7 4 549 THREE LAKES WMA 9 9.3 4 601 LAKE OKEECHOBEE (WEST) 8 11.7 448 602 LAKE OKEECHOBEE (NORTH) 9 1.8 163 603 LAKE OKEECHOBEE (EAST) 8 6.8 38 604 LAKE OKEECHOBEE (SOUTH) 8 5.2 323 711 LAKE HANCOCK 9 3.9 101 721 RODMAN RESERVOIR 8 7.0 118 722 ORANGE LAKE 8 9.3 125 723 LOCHLOOSA LAKE 9 3.4 56 734 LAKE SEMINOLE 9 1.5 16 741 LAKE TRAFFORD -

The Shellcracker
the Shellcracker FLORIDA CHAPTER OF THE AMERICAN FISHERIES SOCIETY http://www.sdafs.org/flafs April, 2011 President’s Message: Greetings from South Florida, folks. For those of you who weren’t able to attend in January, you missed one heck of a Southern Division meeting in Tampa. With 408 attendees and over 200 individual oral and presentations, we’re still getting complements on how well it turned out. I appreciate all of the help that folks around the chapter offered, but special thanks need to be given to Eric Nagid, Linda Lombardi-Carlson, Kerry Flaherty, Andy Strickland, Wes Porak, and the rest of the planning committee for their tireless efforts to make this meeting a success. As many of you know, or perhaps are fast learning, this is a challenging time to be involved in fisheries sci- ence. Federal and state employees are both facing such issues as departmental hiring freezes, field and travel budget reductions, and an increasing individual workload. On the academic side, many colleges and universities are choosing to not back-fill positions upon retirements and are similarly increasing individual workloads on remaining faculty, not to mention the general decrease in external grant and contract funding that served in the past to support many of us and our research in graduate school. We may find our work personally fulfilling, and we often get to see things during our careers that would make many people envious, but I wish sometimes I knew where the public got its perception of the cushy life of the fisheries biologist! Now, it’s one thing to complain and yet another to offer solutions, as many of us likely heard from our parents growing up. -

American Shad Habitat Plan Update
American Shad Habitat Plan Update State of Florida Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute Division of Marine Fisheries Management Reid Hyle [email protected] April 2021 Approved May 5, 2021 Introduction Amendment 3 to the Interstate Management Plan for Shad and River Herring cites habitat loss and degradation as major factors in the decline of and continued depression of populations of American Shad along the Atlantic coast and requires member states to develop habitat plans for American Shad in their jurisdiction. This plan is submitted to serve as the required habitat plan for the State of Florida. It outlines historic and current habitats available to American Shad in Florida and identifies known threats to those habitats as well as efforts to mitigate those threats. The primary spawning run of American shad in Florida historically was and currently is in the St. Johns River. The only other river lying within Florida in which spawning has been documented historically (Williams and Bruger 1972) and recently (Holder et al. 2011, Dutterer et al. 2011) is the Econlockhatchee River which is a tributary to the St. Johns River. The St. Marys River is along the eastern border between Georgia and Florida historically supported a population of American Shad. This plan includes these three systems. The Ocklawaha River is the largest tributary of the St. Johns River and is the largest Atlantic drainage river in Florida obstructed by a dam in its lower reaches. There is no record of a spawning run of American Shad in the Ocklawaha River pre-dating construction of the dam in 1968. -
U N S U U S E U R a C S
Canaveral Natl VOLUSIA Seash Lake Dora Mount Dora O r a Midway Gopher n Wekiva River Slough g Lake Harris e Sanford B Heathrow l o Crystal o s d s Lake n o a m l John F Kennedy Lake Mary r Lake Dr Space Ctr O Tangerine T Harney 108th CongressLake Ola rl of the United States Little Lake Geneva Harris Mosquito Lagoon Lake Jessup StHwy 46 e Zellwood t Howey-in-the-Hills 6 Indian River R Astatula t 2 S 4 S tH DISTRICT 7 w Jordan Slough y 3 Turkey Lake Apopka Wekiwa Springs Longwood r ) John F Kennedy StHwy 414 a D ev Space Ctr Lake Brantley en Buck Lake (G SEMINOLE Mims 4 26 Rte St Winter Springs Puzzle Lake Max Hoeck 1 Creek StRd 46 (Main St) Forest City Casselberry Oviedo Bear Salt Lake StHwy 434 South Altamonte Fern Lake StHwyStHwy 406 StHwy 402 (Max Apopka Springs Park St Johns River StHwy 417 Chuluota 402 Brewer Memorial Pkwy) Canaveral Atlantic Ocean StHwy19 Lake Howell Mills Max Brewer Memorial Pkwy Lake Loughman Lake Natl Seash Lake Arthur Ferndale Maitland Schoolhouse Lake Lockhart Cape Rd Bear Gully Lake South StHwy 406 Lake Apopka Eaton- Clark Lake Lake Paradise Heights ville ( Garden St) W Shepherd a Fairview s Lake Lake h Banana Goldenrod i (Rouse Rd) (Rouse n Grassy DISTRICT Lake Shores 434 StHwy t) Creek Montverde Pickett S Lake Orlando Lake Osceola g th t u o 3 o n Mascotte Fox Lake S l ( A Pintail a ) v r 5 Creek t y e Lake n a 0 Lake 4 Lucy Cherry Lake Minneola Pine Winter Park e w Fairview C n y Lake ( e Sue 8 re w Hills Mills Ave 0 H StHwy 537 4 G t Lake ( 4 M DISTRICT S a y Titusville d Minneola a Lake w i r i n Starke -
F L O R I D a Atlantic Ocean
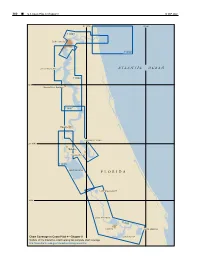
300 ¢ U.S. Coast Pilot 4, Chapter 9 19 SEP 2021 81°30'W 81°W 11491 Jacksonville 11490 DOCTORS LAKE ATL ANTIC OCEAN 11492 30°N Green Cove Springs 11487 Palatka CRESCENT LAKE 29°30'N Welaka Crescent City 11495 LAKE GEORGE FLORIDA LAKE WOODRUFF 29°N LAKE MONROE 11498 Sanford LAKE HARNEY Chart Coverage in Coast Pilot 4—Chapter 9 LAKE JESUP NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 19 SEP 2021 U.S. Coast Pilot 4, Chapter 9 ¢ 301 St. Johns River (1) (8) ENCs - US5FL51M, US5FL57M, US5FL52M, US- Fish havens 5FL53M, US5FL84M, US5FL54M, US5FL56M (9) Numerous fish havens are eastward of the entrance to Charts - 11490, 11491, 11492, 11487, 11495, St. Johns River; the outermost is about 31 miles eastward 11498 of St. Johns Light. (10) (2) St. Johns River, the largest in eastern Florida, is Prominent features about 248 miles long and is an unusual major river in (11) St. Johns Light (30°23'10"N., 81°23'53"W.), 83 that it flows from south to north over most of its length. feet above the water, is shown from a white square tower It rises in the St. Johns Marshes near the Atlantic coast on the beach about 1 mile south of St. Johns River north below latitude 28°00'N., flows in a northerly direction jetty. A tower at Jacksonville Beach is prominent off and empties into the sea north of St. Johns River Light in the entrance, and water tanks are prominent along the latitude 30°24'N.