Paropsine Chrysomelid Attack on Plantations of Eucalyptus Nitens in Tasmania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paropsine Beetles (Coleoptera: Chrysomelidae) in South-Eastern Queensland Hardwood Plantations: Identifying Potential Pest Species
270 Paropsine beetles in Queensland hardwood plantations Paropsine beetles (Coleoptera: Chrysomelidae) in south-eastern Queensland hardwood plantations: identifying potential pest species Helen F. Nahrung1,2,3 1School of Natural Resource Sciences, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia; and 2Horticulture and Forestry Science, Queensland Department of Primary Industries and Fisheries, Gate 3, 80 Meiers Road, Indooroopilly, Queensland 4068, Australia 3Email: [email protected] Revised manuscript received 17 May 2006 Summary The expansion of hardwood plantations throughout peri-coastal Australia, often with eucalypt species planted outside their native Paropsine chrysomelid beetles are significant defoliators of ranges (e.g. E. globulus Labill. in Western Australia; E. nitens Australian eucalypts. In Queensland, the relatively recent (Deane and Maiden) Maiden in Tasmania), resulted in expansion of hardwood plantations has resulted in the emergence unpredicted paropsine species emerging as pests. For example, of new pest species. Here I identify paropsine beetles collected C. agricola (Chapuis) was not considered a risk to commercial from Eucalyptus cloeziana Muell. and E. dunnii Maiden, two of forestry but became a significant pest of E. nitens in Tasmania the major Eucalyptus species grown in plantations in south-eastern (de Little 1989), and the two most abundant paropsine species Queensland, and estimate the relative abundance of each (C. variicollis (Chapuis) and C. nobilitata (Erichson)) in paropsine species. Although I was unable to identify all taxa to E. globulus plantations in WA were not pests of native forest species level, at least 17 paropsine species were collected, about there (compare Selman 1994; Loch 2005), nor were they initially one-third of which have not been previously associated with considered pests of E. -

Coleoptera: Chrysomelidae) and the Paropsine Threat to Eucalyptus in New Zealand
Biological Control of Paropsis charybdis Stål (Coleoptera: Chrysomelidae) and the Paropsine Threat to Eucalyptus in New Zealand A Thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy in the University of Canterbury by Brendan Dene Murphy New Zealand School of Forestry University of Canterbury 2006 TABLE OF CONTENTS ABSTRACT v ACKNOWLEDGEMENTS vi ERRATA vii CHAPTERS Chapter 1. Biological Control of Paropsis charybdis Stål and the Paropsine Threat to Eucalyptus in New Zealand.................................................................................................... 1 Chapter 2. The Collection, Importation, and Release of Tasmanian Enoggera nassaui for Biological Control of Paropsis charybdis............................................................................. 8 Chapter 3. Molecular Detection of Enoggera nassaui Strains using the Mitochondrial DNA Gene, Cytochrome Oxidase I ............................................................................................... 22 Chapter 4. Field and Bioassay Assessment of the Host Range .................................................. 32 Chapter 5. Phylogenetic Reconstruction of Tasmanian Chrysophtharta ..................................45 Chapter 6. Assessment of Paropsine Fecundity as an Indicator................................................. 59 Chapter 7. Testing the Parasitoid Host Range and Reproductive Output Hypotheses against Dicranosterna semipunctata ............................................................................................... -

Biological Control of Paropsis Charybdis
No. 227, July 2012 ISSN 1175-9755 BIOLOGICAL CONTROL OF PAROPSIS CHARYBDIS Paropsine beetles (Coleoptera: Chrysomelidae) is not always easy to locate in Tasmania) from which we are extremely diverse and abundant in their native could obtain larvae for experiments. Australian range but have emerged as significant defoliators only since the expansion of managed The next step was to locate Eadya paropsidis. Flying adults plantation forestry, particularly when host trees are were caught “on-the–wing” in E. nitens plantations in planted outside their native range. Since its arrival in northern Tasmania in December 2011 and brought back New Zealand in 1916 Paropsis charybdis has effectively to the laboratory in Hobart for testing. Using a sequential prevented the commercial viability of several favoured no-choice testing method to observe individual females, 9 Eucalyptus species, including Eucalyptus nitens, until the of 10 of the female wasps attacked P. agricola larvae, then successful introduction of the egg parasitoid Enoggera 7 of those 9 also attacked P. charybdis larvae. nassaui (Hymenoptera: Pteromalidae) in 1988. Scion entomologists have been involved intermittently in the Those P. charybdis larvae attacked were quickly shown search for classical biological control agents for Paropsis to be a suitable physiological host for E. paropsidis charybdis for nearly fifty years, and this appears set to development; parasitoid larvae emerged from the continue for at least another two years. paropsine larvae they had killed, and were significantly larger from P. charybdis than from P. agricola. Paropsis charybdis is bivoltine in New Zealand. The Unfortunately over the whole experiment only 8% first generation of eggs are laid in spring from October of E. -
To Obtain Approval for New Organisms in Containment
APPLICATION FORM Containment To obtain approval for new organisms in containment Send to Environmental Protection Authority preferably by email ([email protected]) or alternatively by post (Private Bag 63002, Wellington 6140) Payment must accompany final application; see our fees and charges schedule for details. Application Number APP203338 Date 28 July 2017 www.epa.govt.nz 2 Application Form Approval for new organism in containment Completing this application form 1. This form has been approved under section 40 of the Hazardous Substances and New Organisms (HSNO) Act 1996. It only covers importing, development (production, fermentation or regeneration) or field test of any new organism (including genetically modified organisms (GMOs)) in containment. If you wish to make an application for another type of approval or for another use (such as an emergency, special emergency or release), a different form will have to be used. All forms are available on our website. 2. If your application is for a project approval for low-risk GMOs, please use the Containment – GMO Project application form. Low risk genetic modification is defined in the HSNO (Low Risk Genetic Modification) Regulations: http://www.legislation.govt.nz/regulation/public/2003/0152/latest/DLM195215.html. 3. It is recommended that you contact an Advisor at the Environmental Protection Authority (EPA) as early in the application process as possible. An Advisor can assist you with any questions you have during the preparation of your application including providing advice on any consultation requirements. 4. Unless otherwise indicated, all sections of this form must be completed for the application to be formally received and assessed. -

100 Years of the Eucalyptus Tortoise Beetle in New Zealand Toni Withers and Elise Peters
Professional papers 100 years of the eucalyptus tortoise beetle in New Zealand Toni Withers and Elise Peters Abstract Arrival and spread The eucalyptus tortoise beetle, Paropsis charybdis, The eucalyptus tortoise beetle, Paropsis charybdis, has been one of the most successful insect pests to was first located at Coopers Knob on Banks Peninsula in invade New Zealand. One hundred years have now 1916 (Thomson, 1922). Clark (1938) speculated that ‘the passed, and yet this pest continues to cause anxiety insect was probably imported in the egg or early larval to forest managers and impact the growth of eucalypt stages upon young eucalypt plants, although possibly plantations. Research shows there are serious cost hibernating adults may have entered the country implications for plantation managers if they neglect under the bark of imported Australian hardwoods.’ All to manage tortoise beetle outbreaks across multiple we know for sure is that the pest established quickly seasons. Many attempts have been made to control in Canterbury. Those founding individuals arrived this insect pest with biological control agents imported without any of the natural enemies that regulate their from its native Australia, with some success. number in Australia. Scion is hoping to introduce yet another natural In New Zealand, the only insects since observed enemy in 2018, a braconid parasitoid that targets the attacking tortoise beetle larvae are some predatory larval life stage. The importance of integrating aerial pentatomids (bugs) (Valentine, 1967). So with spray technologies with biological control agents to conditions perfect for its rapid population growth, minimise negative impacts on these beneficial insects the eucalyptus tortoise beetle spread steadily through cannot be under-estimated. -

Biological Control of Gonipterus Platensis
BIOLOGICAL CONTROL OF GONIPTERUS PLATENSIS: CURRENT STATUS AND NEW POSSIBILITIES CARLOS MANUEL FERREIRA VALENTE ORIENTADORA: Doutora Manuela Rodrigues Branco Simões TESE ELABORADA PARA OBTENÇÃO DO GRAU DE DOUTOR EM ENGENHARIA FLORESTAL E DOS RECURSOS NATURAIS 2018 BIOLOGICAL CONTROL OF GONIPTERUS PLATENSIS: CURRENT STATUS AND NEW POSSIBILITIES CARLOS MANUEL FERREIRA VALENTE ORIENTADORA: Doutora Manuela Rodrigues Branco Simões TESE ELABORADA PARA OBTENÇÃO DO GRAU DE DOUTOR EM ENGENHARIA FLORESTAL E DOS RECURSOS NATURAIS JÚRI: Presidente: Doutora Maria Teresa Marques Ferreira Professora Catedrática Instituto Superior de Agronomia Universidade de Lisboa Vogais: Doutora Maria Rosa Santos de Paiva Professora Catedrática Faculdade de Ciências e Tecnologia Universidade Nova de Lisboa; Doutora Manuela Rodrigues Branco Simões Professora Auxiliar com Agregação Instituto Superior de Agronomia Universidade de Lisboa; Doutor José Carlos Franco Santos Silva Professor Auxiliar Instituto Superior de Agronomia Universidade de Lisboa; Doutor Edmundo Manuel Rodrigues de Sousa Investigador Auxiliar Instituto Nacional de Investigação Agrária e Veterinária. 2018 À Susana e à Leonor i Em memória da minha Avó, Maria dos Anjos Valente (1927-2017) ii Agradecimentos Agradeço, em primeiro lugar, à Professora Manuela Branco, pelo apoio incansável na orientação desta tese, a total disponibilidade e os inúmeros ensinamentos. Ao RAIZ, pelo financiamento do doutoramento, e à sua Direção, em particular ao Engenheiro Serafim Tavares, ao Engenheiro José Nordeste, ao Professor Carlos Pascoal Neto, à Engenheira Leonor Guedes, ao Gabriel Dehon e ao Nuno Borralho, pelo voto de confiança e incentivo que sempre me transmitiram. Deixo um especial agradecimento à Catarina Gonçalves e à Catarina Afonso, pela amizade, por terem ajudado a manter os projetos do RAIZ e a biofábrica a funcionar, pelas horas infindáveis passadas no laboratório e pelos excelentes contributos científicos que muito melhoraram a qualidade desta tese. -

Paropsis Charybdis Defoliation of Eucalyptus Stands in New Zealand’S Central North Island
Forestry 334 PAROPSIS CHARYBDIS DEFOLIATION OF EUCALYPTUS STANDS IN NEW ZEALAND’S CENTRAL NORTH ISLAND B.D. MURPHY1 and M.K. KAY2 1NZ School of Forestry, University of Canterbury, Private Bag 4800, Christchurch 2 Forest Research, Private Bag 3020, Rotorua ABSTRACT Paropsis charybdis, the most serious pest of Eucalyptus in New Zealand, was controlled with the introduced Australian egg parasitoid Enoggera nassaui in the late 1980s. Using frass traps to monitor P. charybdis populations, we report that pest outbreaks still occur, resulting in heavy defoliation of susceptible Eucalyptus species. The results suggest that the presence of large larval populations and commensurate defoliation result from poor spring parasitism by the parasitoid. A second wave of P. charybdis oviposition is effectively attacked, preventing late season defoliation by larvae. A climatic mismatch of E. nassaui is suspected to be the cause of this poor performance. Keywords: Paropsis charybdis, Enoggera nassaui, Eucalyptus, biological control. INTRODUCTION The Australian Eucalyptus tortoise beetle, Paropsis charybdis Stål (Coleoptera: Chrysomelidae), is a highly fecund, bivoltine species that accidentally established in New Zealand in the early 1900s (Styles 1970). The lack of natural enemies to regulate this pest resulted in large populations, with both adult and larval stages defoliating susceptible host species. This seriously impeded establishment of a commercially viable New Zealand Eucalyptus resource (Wilcox 1980). Initial success with a classical biological control agent, the egg parasitoid Enoggera nassaui Girault (Hymenoptera: Pteromalidae) from western Australia (Kay 1990), suppressed P. charybdis populations, allowing establishment of susceptible host species, particularly Shining gum, Eucalyptus nitens (Deane & Maiden) Maiden, a tree suitable for short rotation short-fibre pulp production. -

TAXON:Eucalyptus Nicholii Maiden & Blakely SCORE
TAXON: Eucalyptus nicholii Maiden SCORE: -1.0 RATING: Low Risk & Blakely Taxon: Eucalyptus nicholii Maiden & Blakely Family: Myrtaceae Common Name(s): narrow-leaf black peppermint Synonym(s): Eucalyptus acaciiformis var. linearis H.Deane & Maiden narrow-leaf peppermint small-leaf peppermint willow peppermint willow-leaf peppermint Assessor: Chuck Chimera Status: Assessor Approved End Date: 16 Dec 2020 WRA Score: -1.0 Designation: L Rating: Low Risk Keywords: Temperate Tree, Naturalized Elsewhere, Non-toxic, Wind-Dispersed. Coppices Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) Intermediate tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 n subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed -
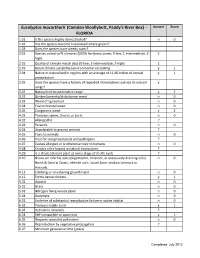
Eucalyptus Macarthurii
Eucalyptus macarthurii (Camden Woollybutt, Paddy's River Box) -- Answer Score FLORIDA 1.01 Is the species highly domesticated? n 0 1.02 Has the species become naturalised where grown? 1.03 Does the species have weedy races? 2.01 Species suited to FL climates (USDA hardiness zones; 0-low, 1-intermediate, 2- 2 high) 2.02 Quality of climate match data (0-low; 1-intermediate; 2-high) 2 2.03 Broad climate suitability (environmental versatility) y 1 2.04 Native or naturalized in regions with an average of 11-60 inches of annual y 1 precipitation 2.05 Does the species have a history of repeated introductions outside its natural y range? 3.01 Naturalized beyond native range y 2 3.02 Garden/amenity/disturbance weed n 0 3.03 Weed of agriculture n 0 3.04 Environmental weed n 0 3.05 Congeneric weed y 2 4.01 Produces spines, thorns or burrs n 0 4.02 Allelopathic ? 4.03 Parasitic n 0 4.04 Unpalatable to grazing animals ? 4.05 Toxic to animals n 0 4.06 Host for recognised pests and pathogens ? 4.07 Causes allergies or is otherwise toxic to humans n 0 4.08 Creates a fire hazard in natural ecosystems ? 4.09 Is a shade tolerant plant at some stage of its life cycle ? 4.10 Grows on infertile soils (oligotrophic, limerock, or excessively draining soils). n 0 North & Central Zones: infertile soils; South Zone: shallow limerock or Histisols. 4.11 Climbing or smothering growth habit n 0 4.12 Forms dense thickets y 1 5.01 Aquatic n 0 5.02 Grass n 0 5.03 Nitrogen fixing woody plant n 0 5.04 Geophyte n 0 6.01 Evidence of substantial reproductive failure in native -

Coleoptera: Chrysomelidae) in a Semi-Rural Suburb in New Zealand Emmanuel Yamoah1*, Dave Voice2, Disna Gunawardana3, Brad Chandler1 and Don Hammond4
Yamoah et al. New Zealand Journal of Forestry Science (2016) 46:5 DOI 10.1186/s40490-016-0061-3 SHORT REPORT Open Access Eradication of Paropsisterna beata (Newman) (Coleoptera: Chrysomelidae) in a semi-rural suburb in New Zealand Emmanuel Yamoah1*, Dave Voice2, Disna Gunawardana3, Brad Chandler1 and Don Hammond4 Abstract Background: A large population of Paropsisterna beata (eucalyptus leaf beetle) was detected on Eucalyptus nitens (H. Deane & Maiden) Maiden (Myrtaceae) at Whitemans Valley, a suburb east of Upper Hutt, Wellington, in 2012. The suburb is a semi-rural residential area with a large number of eucalypt, planted for amenity, shelterbelt and firewood. Surveillance to delimit spread showed that the beetle population was confined to about 0.7 ha consisting of about 40 eucalypts. The Ministry for Primary Industries (MPI) initiated a response to eradicate the beetle population. Findings: Aerial applications of Dominex EC 100 (alpha-cypermethrin) and ground applications of Talstar (bifenthrin) respectively over a 15-month period targeted the adults and larvae in the foliage and the pre-pupae, larvae and emerging adults in the leaf litter. Removal of overwintering habitat by stripping loose bark from host trees further reduced the beetle population. Following these treatments, the beetle has not been detected through a series of surveys using light traps, bark inspection, sticky tapes, visual inspection from the ground, climbing and felling host trees for inspection for 2 years since the last detection of two adults on neighbouring trees. Conclusions: The P. beata population has been successfully eradicated using a combination of aerial and ground- based application of insecticides. -

HTHF Technote
Date: 16 May 2018 Reference: Sidney 60633 Laboratory tests to determine if an Australian wasp, Eadya daenerys, is suitable for biological control of the Eucalyptus tortoise beetle, Paropsis charybdis Author: Toni Withers Corresponding author: [email protected] Summary: We conducted extensive laboratory host-range tests with female Eadya daenerys against the target pest Paropsis charybdis and also against nine other closely-related beetle species found in New Zealand. These allow us to predict how the Australian parasitoid might behave in New Zealand, and the possible consequences and potential risks it poses to other non-target beetle species. Introduction The Australian Eucalyptus tortoise beetle, Paropsis charybdis, is a major pest within New Zealand gum plantations. Present for over 100 years (Withers & Peters 2017), the pest has caused damage, defoliation, and sometimes death, to many gum trees throughout the country (Bain & Kay 1989). Paropsis charybdis finds some species of gum trees to be particularly palatable, especially Eucalyptus. nitens (shining gum), a species grown mainly for wood and pulp and paper making (Murphy & Kay 2000), and other Eucalyptus species being grown for ground durable-wood and lumber production (Lin et al. 2017). The pest is responsible for economic losses within the entire forest products industry. To manage the pest population of Paropsis Eucalyptus tortoise beetle larvae, the target pest charybdis, chemical control with aerial spraying of insecticides occurs in up to a quarter of large plantations annually. However, the costs associated with aerial spraying are prohibitive for many growers and a major barrier to increasing eucalypt plantations (Withers et al. 2013). Other undesirable outcomes, such as environmental and ecological harm and risking FSC certification could also result from long- term use of chemical insecticides. -

Eucalyptus Variegated Beetle Creates Concern for Eucalypt Growers
Concern for eucalypts Eucalyptus variegated beetle creates concern for eucalypt growers Toni Withers, Rebecca McDougal, Michelle Harnett and Tara Murray Annoying Australians are over here and eating ‘our’ eucalypt trees. Seven different tortoise beetles, named because their round bodies resemble a tortoise, have arrived in New Zealand and have been making pests of themselves for more than 100 years. The most recent arrival is Paropsisterna variicollis, the species in the sub-genus Symphyomyrtus, particularly eucalyptus variegated beetle. Its presence was noted E. camaldulensis river red gum, E. viminalis ribbon gum, in Hawke’s Bay in 2016. A fast lifecycle, at least three E. globulus blue gum and E. nitens. It and has been the generations a year, clustered larval feeding, a high focus of four biological control introductions since reproductive output, and the fact that it feeds on both the 1970s. It is also the only production forestry insect juvenile and adult leaves means eucalyptus variegated pest controlled by repeated aerial sprays in attempts beetle is an unwelcome incursion. Furthermore, it to maintain the growth rate of E. nitens plantations. is a beetle with the potential to affect a wide range Eucalyptus tortoise beetle is also a significant defoliator of eucalypts, and where conditions support multiple of E. quadrangulata, one of the species of interest to the generations each year, it may have potential to cause NZ Dryland Forests Initiative. repeated defoliation and affect growth. Unfortunately, the known biology of eucalyptus Eucalyptus variegated beetle has spread throughout variegated beetle suggests it is a strong contender to Hawke’s Bay and in 2018 moved to north east Hawke’s knock P.