Heteroptera: Nabidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Venoms of Heteropteran Insects: a Treasure Trove of Diverse Pharmacological Toolkits
Review Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits Andrew A. Walker 1,*, Christiane Weirauch 2, Bryan G. Fry 3 and Glenn F. King 1 Received: 21 December 2015; Accepted: 26 January 2016; Published: 12 February 2016 Academic Editor: Jan Tytgat 1 Institute for Molecular Biosciences, The University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (G.F.K.) 2 Department of Entomology, University of California, Riverside, CA 92521, USA; [email protected] (C.W.) 3 School of Biological Sciences, The University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (B.G.F.) * Correspondence: [email protected]; Tel.: +61-7-3346-2011 Abstract: The piercing-sucking mouthparts of the true bugs (Insecta: Hemiptera: Heteroptera) have allowed diversification from a plant-feeding ancestor into a wide range of trophic strategies that include predation and blood-feeding. Crucial to the success of each of these strategies is the injection of venom. Here we review the current state of knowledge with regard to heteropteran venoms. Predaceous species produce venoms that induce rapid paralysis and liquefaction. These venoms are powerfully insecticidal, and may cause paralysis or death when injected into vertebrates. Disulfide- rich peptides, bioactive phospholipids, small molecules such as N,N-dimethylaniline and 1,2,5- trithiepane, and toxic enzymes such as phospholipase A2, have been reported in predatory venoms. However, the detailed composition and molecular targets of predatory venoms are largely unknown. In contrast, recent research into blood-feeding heteropterans has revealed the structure and function of many protein and non-protein components that facilitate acquisition of blood meals. -

New Records of Heteroptera (Hemiptera) Species from Turkey, with Reconsideration of Several Previous Records
Use the following type of citation: North-western Journal of Zoology 2021: e201203 Paper Submitted to The North-Western Journal of Zoology 1 *Handling editor: Dr. H. Lotfalizadeh 2 *Manuscript Domain: Entomology 3 *Manuscript code: nwjz-20-EN-HL-07 4 *Submission date: 21 September 2020 5 *Revised: 12 December 2020 6 *Accepted: 12 December 2020 7 *No. of words (without abstract, acknowledgement, references, tables, captions): 7431 8 (papers under 700 words are not accepted) 9 *Editors only: 10 11 Zoology 12 Title of the paper: New records of Heteroptera (Hemiptera)of species from Turkey, with 13 reconsideration of several previous records proofing 14 Journaluntil 15 Running head: New Records of Heteroptera from Turkey 16 paper 17 Authors (First LAST - without institution name!): Barış ÇERÇİ, Serdar TEZCAN 18 North-western 19 Accepted 20 Key Words (at least five keywords): New records, previous records, Nabidae, Reduviidae, Miridae, 21 Turkey 22 23 24 No. of Tables: 0 25 No. of Figures: 4 26 No. of Files (landscape tables should be in separate file): 0 Use the following type of citation: North-western Journal of Zoology 2021: e201203 nwjz-2 27 New records of Heteroptera (Hemiptera) species from Turkey, with reconsideration of 28 several previous records 29 Barış, ÇERÇİ1, Serdar, TEZCAN2 30 1. Faculty of Medicine, Hacettepe University, Ankara, Turkey 31 2. Department of Plant Protection, Faculty of Agriculture, Ege University, Izmir, Turkey 32 * Corresponding authors name and email address: Barış ÇERÇİ, 33 [email protected] 34 35 Abstract. In this study, Acrotelus abbaricus Linnavuori, Dicyphus (Dicyphus) josifovi Rieger, 36 Macrotylus (Macrotylus) soosi Josifov, Myrmecophyes (Myrmecophyes) variabilis Drapulyok Zoology 37 and Paravoruchia dentata Wagner are recorded from Turkey for the first time. -

DAMSEL BUGS Hemiptera: Nabidae Nabis Alternatus, N
Modified from Ralph E. Berry. 1998©. Insects and Mites of Economic Importance in the Northwest. 2nd Ed. 221 p. DAMSEL BUGS Hemiptera: Nabidae Nabis alternatus, N. americoferus ___________________________________________________________________________ DESCRIPTION Adults are tan or grayish-brown, with piercing- sucking mouthparts and well-developed wings. The membranous portion of the first pair of wings has a number of small cells around the margin. The front pair of legs is modified for grasping their prey. They have slender bodies, and are about 4 to 12 mm long. Nymphs resemble adults, except they are smaller and have no wings. LIFE HISTORY Western damsel bug adult feeding on lygus Damsel bugs overwinter as adults in protected places around the margins of fields. Adults of the Western damsel bug, N. alternatus, begin emerging in May or early June and N. americolferus begins emerging in late March or early April. Adults begin laying eggs soon after emergence. Eggs are flattened on top and are inserted into soft plant tissue along the stems. Eggs hatch into nymphs, which feed on small insects, mites, or eggs. Early instar nymphs may be found on the soil surface beneath plants and under litter near the stems. N. americolferus has two complete Western Damsel bug nymph generations each year with little overlap between the develoopmental stages. N. alternatus has numerous, overlapping generations during the season. DAMSEL BUG IMPORTANCE ADULTS EGGS, NYMPHS, ADULTS Adults and nymphs feed on many soft-bodied insects, OVERLAPPING GENERATIONS including aphids, spider mites, leafhoppers, small ADULTS caterpillars, and insect eggs including those of the J F M A M J J A S O N D Colorado potato beetle. -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -

USING NEW MOLECULAR TOOLS to EXPLORE HOW ORGANIC FARMING IMPACTS TOP-DOWN and BOTTOM-UP REGULATION of POTATO HERBIVORES by KARO
USING NEW MOLECULAR TOOLS TO EXPLORE HOW ORGANIC FARMING IMPACTS TOP-DOWN AND BOTTOM-UP REGULATION OF POTATO HERBIVORES By KAROL LYNN KREY A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Entomology JULY 2017 © Copyright by KAROL LYNN KREY, 2017 All Rights Reserved © Copyright by KAROL LYNN KREY, 2017 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of KAROL LYNN KREY find it satisfactory and recommend that it be accepted. William E. Snyder, Ph.D., Chair David W. Crowder, Ph.D. John P. Reganold, Ph.D. Paul D. Nabity, Ph.D. ii ACKNOWLEDGMENT I would like to thank Bill Snyder, my advisor and committee chair, for the opportunity to be a part of the lab and for his help throughout my graduate career. I have made leaps and bounds in progress toward becoming a great researcher, writer, and mentor. I would also like to thank my committee members Dave Crowder, John Reganold, and Paul Nabity, for providing me guidance and constructive criticisms on my dissertation projects. I am grateful for the many friends and comrades I have made in the entomology department and hope to always stay in touch. I am lucky to have had the support from the Snyder lab team, especially, Christine Lynch, Amanda Meadows, Carmen Castillo, Jake Asplund, Matt Jones, Joseph Taylor, Olivia Smith, and the super amazing post-docs, Daisy Fu and Carmen Blubaugh (your help and support was invaluable). Thanks to all the undergraduate workers that spent tireless hours helping me: Abbey Estep, Ashley Norberg, Trevor Snodgrass, Jen Madigan, and Samantha Beck. -

Arthropod Communities and Transgenic Cotton in the Western United States: Implications for Biological Control S.E
284 Naranjo and Ellsworth ___________________________________________________________________ ARTHROPOD COMMUNITIES AND TRANSGENIC COTTON IN THE WESTERN UNITED STATES: IMPLICATIONS FOR BIOLOGICAL CONTROL S.E. Naranjo1 and P.C. Ellsworth2 1U.S. Department of Agriculture, Agricultural Research Service, Phoenix, Arizona, U.S.A. 2University of Arizona, Maricopa, Arizona, U.S.A. INTRODUCTION Cotton, transgenically modified to express the insecticidal proteins of Bacillus thuringiensis (Bt), has been available commercially in the United States since 1996. Bt cotton is widely used throughout the cotton belt (Layton et al., 1999), and more than 65% of the acreage in Arizona has been planted to Bt cotton since 1997. In the low desert production areas of Arizona and California, Pectinophora gossypiella (Saunders), the pink bollworm, is the major target of Bt cotton. A number of other lepidopterous species occur in this area, but they are sporadic secondary pests of cotton whose population outbreaks are typically induced by indiscriminate use of broad-spectrum insecticides. As a result of the adoption of Bt-cotton and the coincident introduction and adoption of selective insect growth regulators for suppression of Bemisia tabaci (Gennadius), insecticide usage in Arizona cotton over the past decade as declined from a high of 12.5 applications per acre in 1995 to 1.9 in 1999 (Ellsworth and Jones, 2001). These reductions in insecticide use have broadened opportunities for all biological control approaches in cotton. Beyond concern for the maintenance of susceptibility in target pest populations there also are a number of ecological and environmental questions associated with use of transgenic crops, one of the most prominent being effects on non-target organisms. -
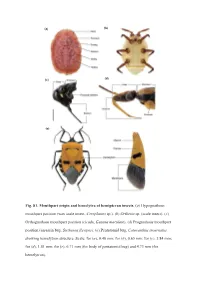
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

First Ever Report of a Bite by Nabis Argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a Human
Short Communication Biodiversity and Natural History (2017) Vol. 3, No. 2, 45-47 First ever report of a bite by Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a human Primer registro de una picadura de Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) en un ser humano Marcela Cornelis1, Fernando Diez1 & María del Carmen Coscarón2 1Universidad Nacional de La Pampa, Facultad de Ciencias Exactas y Naturales, Uruguay 151 L6300CLB, Santa Rosa, La Pampa, Argentina. 2Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo, División Entomología, Paseo del Bosque s/n 1900, La Plata, Buenos Aires, Argentina. *Correspondence author: [email protected] Abstract Herein, we described the first ever reported bite of Nabis argentinus Meyer-Dür 1870 on a human. The bite was registered in the locality Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). The insect was not provoked by the victim, and thus, the bite was probably not in self-defense. We therefore concluded that the insect bite the victim because it was searching for sources of hydration. Key words: adventitious bite, nabids, predaceous habit, record, symptomatology. Resumen Se describe el primer caso de una picadura adventicia en un ser humano por Nabis argentinus Meyer-Dür 1870, en la localidad de Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). El insecto no fue provocado por la víctima, por lo que probablemente la picadura no fue en defensa propia. Por lo tanto, concluimos que el insecto picó a la víctima en búsqueda de recursos de hidratación. -

The Insect Database in Dokdo, Korea: an Updated Version in 2020
Biodiversity Data Journal 9: e62011 doi: 10.3897/BDJ.9.e62011 Data Paper The Insect database in Dokdo, Korea: An updated version in 2020 Jihun Ryu‡,§, Young-Kun Kim |, Sang Jae Suh|, Kwang Shik Choi‡,§,¶ ‡ School of Life Science, BK21 FOUR KNU Creative BioResearch Group, Kyungpook National University, Daegu, South Korea § Research Institute for Dok-do and Ulleung-do Island, Kyungpook National University, Daegu, South Korea | School of Applied Biosciences, Kyungpook National University, Daegu, South Korea ¶ Research Institute for Phylogenomics and Evolution, Kyungpook National University, Daegu, South Korea Corresponding author: Kwang Shik Choi ([email protected]) Academic editor: Paulo Borges Received: 14 Dec 2020 | Accepted: 20 Jan 2021 | Published: 26 Jan 2021 Citation: Ryu J, Kim Y-K, Suh SJ, Choi KS (2021) The Insect database in Dokdo, Korea: An updated version in 2020. Biodiversity Data Journal 9: e62011. https://doi.org/10.3897/BDJ.9.e62011 Abstract Background Dokdo, a group of islands near the East Coast of South Korea, comprises 89 small islands. These volcanic islands were created by an eruption that also led to the formation of the Ulleungdo Islands (located in the East Sea), which are approximately 87.525 km away from Dokdo. Dokdo is important for geopolitical reasons; however, because of certain barriers to investigation, such as weather and time constraints, knowledge of its insect fauna is limited compared to that of Ulleungdo. Until 2017, insect fauna on Dokdo included 10 orders, 74 families, 165 species and 23 undetermined species; subsequently, from 2018 to 2019, we discovered 23 previously unrecorded species and three undetermined species via an insect survey. -

Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises
Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises Michael J. Raupach1*, Lars Hendrich2*, Stefan M. Ku¨ chler3, Fabian Deister1,Je´rome Morinie`re4, Martin M. Gossner5 1 Molecular Taxonomy of Marine Organisms, German Center of Marine Biodiversity (DZMB), Senckenberg am Meer, Wilhelmshaven, Germany, 2 Sektion Insecta varia, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 3 Department of Animal Ecology II, University of Bayreuth, Bayreuth, Germany, 4 Taxonomic coordinator – Barcoding Fauna Bavarica, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 5 Terrestrial Ecology Research Group, Department of Ecology and Ecosystem Management, Technische Universita¨tMu¨nchen, Freising-Weihenstephan, Germany Abstract During the last few years, DNA barcoding has become an efficient method for the identification of species. In the case of insects, most published DNA barcoding studies focus on species of the Ephemeroptera, Trichoptera, Hymenoptera and especially Lepidoptera. In this study we test the efficiency of DNA barcoding for true bugs (Hemiptera: Heteroptera), an ecological and economical highly important as well as morphologically diverse insect taxon. As part of our study we analyzed DNA barcodes for 1742 specimens of 457 species, comprising 39 families of the Heteroptera. We found low nucleotide distances with a minimum pairwise K2P distance ,2.2% within 21 species pairs (39 species). For ten of these species pairs (18 species), minimum pairwise distances were zero. In contrast to this, deep intraspecific sequence divergences with maximum pairwise distances .2.2% were detected for 16 traditionally recognized and valid species. With a successful identification rate of 91.5% (418 species) our study emphasizes the use of DNA barcodes for the identification of true bugs and represents an important step in building-up a comprehensive barcode library for true bugs in Germany and Central Europe as well. -

Comparative Selectivity of Acetamiprid in the Management Of
Pest Management Science Pest Manag Sci 61:555–566 (2005 ) DOI: 10.1002/ps.1030 Conservation of natural enemies in cotton: comparative selectivity of acetamiprid in the management of Bemisia tabaci†‡ Steven E Naranjo∗ and David H Akey USDA-ARS, Western Cotton Research Laboratory, 4135 East Broadway Road, Phoenix, AZ 85040, USA Abstract: The integrated control concept emphasizes the importance of both chemical and biological control for pest suppression in agricultural systems. A two-year field study was conducted to evaluate the selectivity of acetamiprid for the control of Bemisia tabaci (Gennadius) in cotton compared with a proven selective regime based on the insect growth regulators (IGRs) pyriproxyfen and buprofezin. Acetamiprid was highly effective in controlling all stages of B tabaci compared with an untreated control, and generally produced lower pest densities than the IGR regime. Univariate analyses indicated that nine of 17 taxa of arthropod predators were significantly depressed with the use of acetamiprid compared with an untreated control, including common species such as Geocoris punctipes (Say), Orius tristicolor (White), Chrysoperla carnea Stephens sensu lato, Collops vittatus (Say), Hippodamia convergens Guerin-´ Meneville,´ and Drapetis nr divergens. Compared with results from independent, concurrent studies using mixtures of broad-spectrum insecticides at the same research site, acetamiprid depressed populations of fewer predator taxa; but, for eight predator taxa significantly affected by both regimes, the average population reduction was roughly equal. In contrast, only four taxa were significantly reduced in the IGR regime compared with the untreated control and three of these were omnivores that function primarily as plant pests. Principal response curves analyses (a time-dependent, multivariate ordination method) confirmed these patterns of population change for the entire predator community. -

Through Arthropod Eyes Gaining Mechanistic Understanding of Calcareous Grassland Diversity
Through arthropod eyes Gaining mechanistic understanding of calcareous grassland diversity Toos van Noordwijk Through arthropod eyes Gaining mechanistic understanding of calcareous grassland diversity Van Noordwijk, C.G.E. 2014. Through arthropod eyes. Gaining mechanistic understanding of calcareous grassland diversity. Ph.D. thesis, Radboud University Nijmegen, the Netherlands. Keywords: Biodiversity, chalk grassland, dispersal tactics, conservation management, ecosystem restoration, fragmentation, grazing, insect conservation, life‑history strategies, traits. ©2014, C.G.E. van Noordwijk ISBN: 978‑90‑77522‑06‑6 Printed by: Gildeprint ‑ Enschede Lay‑out: A.M. Antheunisse Cover photos: Aart Noordam (Bijenwolf, Philanthus triangulum) Toos van Noordwijk (Laamhei) The research presented in this thesis was financially spupported by and carried out at: 1) Bargerveen Foundation, Nijmegen, the Netherlands; 2) Department of Animal Ecology and Ecophysiology, Institute for Water and Wetland Research, Radboud University Nijmegen, the Netherlands; 3) Terrestrial Ecology Unit, Ghent University, Belgium. The research was in part commissioned by the Dutch Ministry of Economic Affairs, Agriculture and Innovation as part of the O+BN program (Development and Management of Nature Quality). Financial support from Radboud University for printing this thesis is gratefully acknowledged. Through arthropod eyes Gaining mechanistic understanding of calcareous grassland diversity Proefschrift ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann volgens besluit van het college van decanen en ter verkrijging van de graad van doctor in de biologie aan de Universiteit Gent op gezag van de rector prof. dr. Anne De Paepe, in het openbaar te verdedigen op dinsdag 26 augustus 2014 om 10.30 uur precies door Catharina Gesina Elisabeth van Noordwijk geboren op 9 februari 1981 te Smithtown, USA Promotoren: Prof.