Occurance of Low Salinity Water in Mahim Bay 2006
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
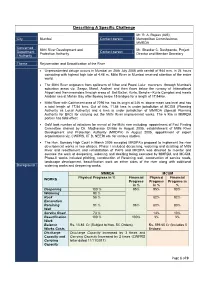
Describing a Specific Challenge
Describing A Specific Challenge Mr. R. A. Rajeev (IAS), City Mumbai Contact person Metropolitan Commissioner, MMRDA Concerned Mithi River Development and Mr. Shankar C. Deshpande, Project Department Contact person Protection Authority Director and Member Secretary / Authority Theme Rejuvenation and Beautification of the River • Unprecedented deluge occurs in Mumbai on 26th July 2005 with rainfall of 944 mm. in 24 hours coinciding with highest high tide of 4.48 m. Mithi River in Mumbai received attention of the entire world. • The Mithi River originates from spillovers of Vihar and Powai Lake traverses through Mumbai's suburban areas viz. Seepz, Marol, Andheri and then flows below the runway of International Airport and then meanders through areas of Bail Bazar, Kurla, Bandra - Kurla Complex and meets Arabian sea at Mahim Bay after flowing below 15 bridges for a length of 17.84Km. • Mithi River with Catchment area of 7295 ha. has its origin at 246 m. above mean sea level and has a total length of 17.84 kms. Out of this, 11.84 kms is under jurisdiction of MCGM (Planning Authority as Local Authority) and 6 kms is under jurisdiction of MMRDA (Special Planning Authority for BKC) for carrying out the Mithi River improvement works. The 6 Km in MMRDA portion has tidal effect. • GoM took number of initiatives for revival of the Mithi river including appointment of Fact Finding Committee chaired by Dr. Madhavrao Chitale in August 2005, establishment of Mithi River Development and Protection Authority (MRDPA) in August 2005, appointment of expert organisations viz. CWPRS, IIT B, NEERI etc. for various studies. -

Notification
NOTIFICATION Whereas, it is considered necessary to ensure smooth flow of vehicular traffic and Agnate processions in Mumbai, I, Brijesh Singh, Addl. Commissioner of Police (Traffic), Mumbai, in exercise of the powers conferred upon me under Section 115 of M.V. Act 1988, (Act No.59 of 1988) read with Government Notification No.MVA 0589/CR 1061/TRA-2, dated 19.05.1990 do hereby order the following traffic regulations on Friday ( 02nd September,) Monday ( 05th September, ) Tuesday ( 06th September, ) Wendsday ( 07th September,) and on Sunday ( 11th September,) 2011, from 12.00 hrs., till 06.00 hrs. (all these days) (I) CLOSURE OF ROADS :- 34 • PYDHUNIE TRAFFIC DIVISION 1. Jeenabhai Mulji Rathod Marg : Will be closed for vehicular traffic, from its Jn. with Shivdas Chapsi Marg to P. D’Mello Road Jn. • KALBADEVI AND TARDEV TRAFFIC DIVISION 2. V. P. Road : From Cawasji Patel Tank to S.V.P. Road Jn. and its Jn. with Dr. Bhadkamkar Marg ( Lamington Road except for vehicles carrying Ganpati Idols and proceeding towards Chowpatty ) shall be closed for vehicular traffic. 3. Jagannath Shanker Sheth Marg (Girgaum Road) : From Princess Street Jn. to S.V.P. Road Jn. (Opera House) will be closed for vehicular traffic. 4. Dr. Jaykar Marg (Thakurdwar Marg) : From its Jn. with Jagannath Shanker Sheth Marg to its Jn. with Maharshi Karve Road (Queen's Road) shall be closed for vehicular traffic. 5. Sardar Vallabhbhai Patel Marg (Sandhurst Road) : (a) Sardar Vallabhbhai Patel Marg :- From its jn. with Dr. N.A. Purandare Marg (Marine Drive) to its Jn. with Pandit Paluskar Chowk (Opera House) and further upto its jn. -
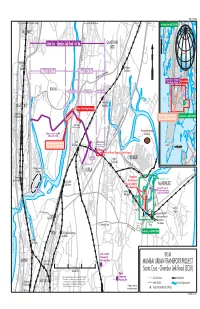
Chembur Link Road (SCLR) Matunga to Mumbai Rail Station This Map Was Produced by the Map Design Unit of the World Bank
IBRD 33539R To Jogeswari-Virkhroli Link Road / Borivali To Jogeswari-Virkhroli Link Road To Thane To Thane For Detail, See IBRD 33538R VILE PARLE ek re GHATKOPAR C i r Santa Cruz - Chembur Link Road: 6.4 km o n a SATIS M Mumbai THANE (Kurla-Andhai Road) Vile Parle ek re S. Mathuradas Vasanji Marg C Rail alad Station Ghatkopar M Y Rail Station N A Phase II: 3.0 km Phase I: 3.4 km TER SW S ES A E PR X Lal Bahadur Shastri Marg WESTERN EXPRESSWAY E Santa Cruz - Chembur Link Road: 6.4 km Area of Map KALINA Section 1: 1.25 km Section 2: 1.55 km Section 3: .6 km ARABIAN Swami Vivekananda Marg SEA Vidya Vihar Thane Creek SANTA CRUZ Rail Station Area of Gazi Nagar Request Mahim Bay Santa Cruz Rail Station Area of Shopkeepers' Request For Detail, See IBRD 33540R For Detail, See IBRD 33314R MIG Colony* (Middle Income Group) Central Railway Deonar Dumping 500m west of river and Ground 200m south of SCLR Eastern Expressway R. Chemburkar Marg Area of Shopkeepers' Request Kurla MHADA Colony* CHURCHGATE CST (Maharashtra Housing MUMBAI 012345 For Detail, See IBRD 33314R Rail Station and Area Development Authority) KILOMETERS Western Expressway Area of Bharathi Nagar Association Request S.G. Barve Marg (East) Gha Uran Section 2 Chembur tko CHEMBUR Rail Station parM ankh urdLink Bandra-Kurla R Mithi River oad To Vashi Complex KURLA nar Nala Deo Permanent Bandra Coastal Regulation Zones Rail Station Chuna Batti Resettlement Rail Station Housing Complex MANKHURD at Mankhurd Occupied Permanent MMRDA Resettlement Housing Offices Govandi Complex at Mankhurd Rail Station Deonar Village Road Mandala Deonarpada l anve Village P Integrated Bus Rail Sion Agarwadi Interchange Terminal Rail Station Mankhurd Mankhurd Correction ombay Rail Station R. -

Bus-Shelter-Advertising.Pdf
1 ONE STOP MARKETING 2 What Are You Looking For? AIRLINE/AIRPORT CINEMA DIGITAL NEWSPAPER RADIO TELEVISION MAGAZINE SERVICES OUTDOOR NON TRADITIONAL 3 Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Powai, Mumbai Suresh Nagar, Mumbai Near L&T, Powai Garden, Powai Military Road Juhu-Versova Link Road ,Bharat Nagar/Petrol Pump Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Juhu, Mumbai VN Purav Marg, Mumbai Juhu S.Parulekar Marg, Traffic Towrds Juhu Bus Station Marathi Vidnyan Parishad, V. N. Purav Road, Chunabhatti Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Andheri East, Mumbai Andheri East, Mumbai International Airport Road, Sahar Road, Ambassador Outside Techno Mall, Jogeshwari Link Road, Behram Hotel Bagh 4 Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Lohar Chawl, Mumbai Lad Wadi, Mumbai Kalbadevi Road ,Princess Street 2 Kalbadevi Road ,Princess Street 1 Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Savarkar Nagar, Mumbai Mahim Nature park, Mumbai Near L&T, Powai Garden, Powai Military Road Dharavi Depot, Dumping Road, Dharavi Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Antop Hill, Mumbai Bharat Nagar, Mumbai Antop Hill, Shaikh Misri Road, Antop Hill Juhu-Versova Link Road ,Bharat Nagar/Petrol Pump 5 Bus Shelter @ INR 35,000/- Per Month Bus Shelter @ INR 35,000/- Per Month Wadala, Mumbai Kurla East, Mumbai Wadala Station, Kidwai Marg, Wadala S.T. Depot (Kurla East), S.T. -

Assessment of Flood Mitigation Measure for Mithi River – a Case Study
International Journal of Civil Engineering and Technology (IJCIET) Volume 7, Issue 3, May–June 2016, pp. 56–66, Article ID: IJCIET_07_03_006 Available online at http://iaeme.com/Home/issue/IJCIET?Volume=7&Issue=3 Journal Impact Factor (2016): 9.7820 (Calculated by GISI) www.jifactor.com ISSN Print: 0976-6308 and ISSN Online: 0976-6316 © IAEME Publication ASSESSMENT OF FLOOD MITIGATION MEASURE FOR MITHI RIVER – A CASE STUDY Rituparna Choudhury, B.M. Patil, Vipin Chandra Bharati Vidyapeeth Deemed University, College of Engineering, Department of Civil Engineering, Pune–43, Maharashtra, India Uday B. Patil, T. Nagendra CWPRS, Khadakwasla, Pune–23, Maharashtra, India ABSTRACT Mumbai city which has an area of 437sq km with a population of 12 million came to an abrupt halt because of the unprecedented rainfall of 944mm during the 24 hours starting on 26th July 2005; with 380mm occurring in just 3 hours between 14:30 to 17:30 and hourly rainfall exceeding 126 mm/hr. This particular event is considered to be an extra-ordinary event. Numerical model study using one dimensional mathematical model HEC-RAS is carried out to simulate unsteady flow in Mithi river with the existing conditions and with the telescopic channelization as suggested by MMRDA for 100 years return period and 6 hours storm duration. The appropriate boundary conditions at the upstream, downstream and the internal boundaries were applied. The results indicated that due to the channelization, the average reduction in the water level is of the order of 20 % to25 % and the increase in the conveyance capacity of Mithi River causing rapid flushing of floods, is found to vary from 23% to 340% which is quite significant compared to existing conditions at various locations along the river. -

Mumbai District
Government of India Ministry of MSME Brief Industrial Profile of Mumbai District MSME – Development Institute Ministry of MSME, Government of India, Kurla-Andheri Road, Saki Naka, MUMBAI – 400 072. Tel.: 022 – 28576090 / 3091/4305 Fax: 022 – 28578092 e-mail: [email protected] website: www.msmedimumbai.gov.in 1 Content Sl. Topic Page No. No. 1 General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 4 1.3 Availability of Minerals. 5 1.4 Forest 5 1.5 Administrative set up 5 – 6 2 District at a glance: 6 – 7 2.1 Existing Status of Industrial Areas in the District Mumbai 8 3 Industrial scenario of Mumbai 9 3.1 Industry at a Glance 9 3.2 Year wise trend of units registered 9 3.3 Details of existing Micro & Small Enterprises and artisan 10 units in the district. 3.4 Large Scale Industries/Public Sector undertaking. 10 3.5 Major Exportable item 10 3.6 Growth trend 10 3.7 Vendorisation /Ancillarisation of the Industry 11 3.8 Medium Scale Enterprises 11 3.8.1 List of the units in Mumbai district 11 3.9 Service Enterprises 11 3.9.2 Potentials areas for service industry 11 3.10 Potential for new MSME 12 – 13 4 Existing Clusters of Micro & Small Enterprises 13 4.1 Details of Major Clusters 13 4.1.1 Manufacturing Sector 13 4.2 Details for Identified cluster 14 4.2.1 Name of the cluster : Leather Goods Cluster 14 5 General issues raised by industry association during the 14 course of meeting 6 Steps to set up MSMEs 15 Annexure - I 16 – 45 Annexure - II 45 - 48 2 Brief Industrial Profile of Mumbai District 1. -

MHI-10 Urbanization in India Indira Gandhi National Open University School of Social Sciences
MHI-10 Urbanization in India Indira Gandhi National Open University School of Social Sciences Block 8 COLONIAL CITIES - 2 UNIT 37 Modernity and the City in Colonial India 5 UNIT 38 City Planning in India under British Rule 19 UNIT 39 Predicaments of Post Colonial Cities 34 UNIT 40 Case Study : Bombay 48 Expert Committee Prof. B.D. Chattopadhyaya Prof. Sunil Kumar Dr. P.K. Basant Formerly Professor of History Department of History Department of History Centre for Historical Studies Delhi University, Delhi Jamia Milia Islamia, New Delhi JNU, New Delhi Prof. Swaraj Basu Prof. Amar Farooqui Prof. Janaki Nair Faculty of History Department of History Centre for Historical Studies IGNOU, New Delhi Delhi University, Delhi JNU, New Delhi Prof. Harbans Mukhia Dr. Vishwamohan Jha Prof. Rajat Datta Formerly Professor of History Atma Ram Sanatan Dharm Centre for Historical Studies Centre for Historical Studies College JNU, New Delhi JNU, New Delhi Delhi University, Delhi Prof. Lakshmi Subramanian Prof. Yogendra Sharma Prof. Abha Singh (Convenor) Centre for Studies in Social Centre for Historical Studies Faculty of History Sciences, Calcutta JNU, New Delhi IGNOU, New Delhi Kolkata Prof. Pius Malekandathil Dr. Daud Ali Centre for Historical Studies South Asia Centre JNU, New Delhi University of Pennsylvania, Philadelphia Block Editor : Prof. Janaki Nair Course Coordinator : Prof. Abha Singh Programme Coordinator : Prof. Swaraj Basu Block Preparation Team Unit No. Resource Person 37 Dr. Prashant Kidambi School of Historical Studies University of Leicester, Leicester, UK. 38 Prof. Howard Spodek Temple University Philadelphia, U.S.A. 39 Dr. Awadhendra Saran Centre for the Study of Developing Societies, University of Delhi, Delhi. -

Bandra Worli Sea Link
Mumbai, Tuesday, June 30, 2009 10 A DNA Promotional Feature in association with HOW THIS IMPRESSIVE EDIFICE WILL CHANGE YOUR COMMUTE Transforming dreams to reality The projected difference in an average road journey from Lovegrove Junction to Mahim Junction THE SCENARIO TILL DATE NOWE WITH THE BANDRA WORLI SEA LINK Facilitating a faster phase it will connect Bandra to HCC (Hindustan Construction The Link Bridge layout is cat- Distance: 7.7 km Via Sealink: 4.70 km Worli where as in the subsequent Company). egorized into three different Traffic Signals: 23 Traffic Signals: 4 commute and phases the plans are to take it fur- Until now, Mahim Causeway, parts: reducing traffic jams ther to Haji Ali and then to Nari- the North-South connector be- Part 1: The north end approach Morning Peak Hour Travel: 35 minutes Morning Peak Hour Travel: 6 minutes man Point. It is a connecting tween the western suburbs and structure mainly with precast Evening Peak Hour Travel: 38 minutes Evening Peak Hour Travel: 6 minutes are the twin goals of bridge linking the city of Mum- the island city of Mumbai getting (PC) segmental construction the Bandra Worli bai with its western suburbs and increasingly bottleneck prone. It Part 2: The Cable Stayed Bridge Average Journey Speed: 13 Kmph Average Journey Speed: 80 Kmph has the potential to bring about takes commuters nearly an hour at Bandra channel is with 50m - Sea Link, which is permanent and far reaching to travel the 8 km distance from 250m-250m-50m span arrange- finally opening up changes in the travel patterns of Mahim to Worli. -

Y8 Mumbai Pack 2 Mumbai Is One of the Most Diverse, Interesting and Dynamic Cities on Our Planet
Y8 Mumbai Pack 2 Mumbai is one of the most diverse, interesting and dynamic cities on our planet. Over the next few weeks you are going to be investigating its culture, location, climate and economy. Instructions This booklet covers 2 main areas about Mumbai: What sports are played in Mumbai and why are they so popular? Why is Mumbai the Film capital of the world and why is Bollywood so successful? Each task might take you between 10 to 30 minutes. At the end of the fortnight a completed answer booklet will be sent out to you and you can use this to mark your work. You may wish to print out the booklet if you want to/can or you may want to complete it on a computer, either is fine. There are different tasks to complete. Please do what you can and don’t panic if you can’t complete something. There’s more work here than you need, so pick the parts that appeal to you the most. We hope you enjoy! Y8 Mumbai Pack 2 Sport and Culture in developing Mumbai Mumbai is one of the most diverse, interesting and dynamic cities on our planet. Over the next few weeks you are going to be investigating its culture, location, climate and economy. 1. 10 mins We have already started to use some key words. To recap and refresh some of the ones we’ve looked at and to introduce some new ones have a go at the word search below. M N M M G Y Z M B C C Q A Z T I O U W A T T O O E A R F N O T I M X Y H M I C N A M E W Q H T B B J B A N C B S M S G H I A A U A I A R I A P O X X I R L I Y N N V A A O G M O B V I U N S I Q N R L S P E R N A V P C F K S N E A G H V M Z R E O T Z E A V G K U K T V W A R P C P H E O T T T T Y R D H Y R H G D Y A B M I H A M A D V E G E J Z T N X S W G P F W C V R B I L L I O N A I R E S W O J T N O R Z U M U L S E R P F E X P O R T S G X Z L Y W ARABIAN SEA FINANCE MONSOON BILLIONAIRES MAHARASHTRA MUMBAI BOMBAY MAHIM BAY OVERPOPULATION DHARAVI MEGACITY REDEVELOPMENT EXPORTS MITHI RIVER SLUM 2. -

Mumbai's Open Spaces Data
MUMBAI’S OPEN SPACES Maps & A Preliminary Listing Document Prepared by Contents Introduction........................................................2 H(W) ward........................................................54 Mumbai's Open Spaces Data..............................4 K(E) ward.........................................................60 Mumbai's Open Spaces Map...............................5 K(W) ward........................................................66 Mumbai's Wards Map..........................................7 P(N) ward.........................................................72 P(S) ward.........................................................78 City - Maps & Open Spaces List ----------------------------------------------------------------- R(N) ward.........................................................84 A ward................................................................8 R(C) ward.........................................................90 B ward..............................................................12 R(S) ward.........................................................96 C ward..............................................................16 D ward..............................................................20 Central & Eastern - Maps & Open Spaces List ----------------------------------------------------------------- E ward..............................................................24 L ward............................................................100 F(N) ward.........................................................30 -

Mahim Bay: a Polluted Environment of Bombay
Mahim Bay: a polluted environment of Bombay Item Type article Authors Govindan, K.; Desai, B.N. Download date 25/09/2021 04:52:42 Link to Item http://hdl.handle.net/1834/31678 Journal of the Indian Fisheries Association, 10 & 11, 1980-81, 5 - 10 MAHIM BAY -A POLLUTED ENVIRONMENT OF BOMBAY K. GOVINDAN & B. N, DESAI. National Institute of Oceanography, Regional Centre, Versova. Bombay-400 061. ABSTRACT Distribution of manne life in relation to the extent of pollution at and outside the Mahim bay was studied. A poor marine fauna at stations A & B was associated with relatively higher intensity of pollution accompanied by higher BOD and nutrients and lower DO levels. A distinct deterioration in the marine life and water quality along the northern part of the bay as compared to the southern part was evident. An incr~:tsing trend in the marine fauna with · decreasing intensity of pollution from inside to outside bay was noticed. INTRODUCTION The Mahim creek is a well known dumping water body for sewage and indus trial waste discharge of the western suburbs of Bombay. About 30 years ago Mahim Bay (Lat l9°2'N, Long 72o49'E) was considered one of the most important fishery centre for Clams and rock oysters ( Subrahmanyam~ Karandikar and. Murti,. 1949), Today fishing activities within the •Mahim: bay have been br<:mght down to nil due to reducing fishery potential ·'(Gajbhiye 1982) with increasing pollution (Zingde and Desai. ·t980) of the bay waters. Marine life in bay is· very weagre today. This ecological change is mainly brought about by the discharges of about 185 million litre per day (MLD) of sewage and industrial effluents. -

33-Second Management Response (Gazi Nagar)
BANK MANAGEMENT RESPONSE TO REQUEST FOR INSPECTION PANEL REVIEW OF THE INDIA – MUMBAI URBAN TRANSPORT PROJECT (IBRD LOAN No. 4665-IN; IDA CREDIT No. 3662-IN) Management has reviewed the Request for Inspection of the India – Mumbai Urban Transport Project (IBRD Loan No. 4665-IN; IDA Credit No. 3662-IN), received by the Inspection Panel on June 24, 2004 and registered on June 29, 2004 (RQ04/4). Manage- ment has prepared the following response. CONTENTS Abbreviations and Acronyms ......................................................................................... iv I. Introduction.............................................................................................................. 1 II. The Request .............................................................................................................. 2 III. Project Background................................................................................................. 4 Project Context........................................................................................................ 6 Resettlement Under the Project .............................................................................. 7 IV. Issues Associated with the Santa Cruz-Chembur Link Road............................ 10 V. Resolving Requesters’ Concerns and Improving Resettlement Implementation............................................................ 13 Actions Underway and Planned: July through August 2004................................ 14 Medium-Term Actions to Enhance Overall Capacity to