Atoms and Molecules Ebook, Epub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Particle Physics 4Th Edition Free Ebook
PARTICLE PHYSICS 4TH EDITION DOWNLOAD FREE BOOK B R Martin | --- | --- | --- | 9781118912294 | --- | --- Particle physics Archived from the original on 1 February That term was deprecated [ citation needed ] after the formulation of the Standard Model during the s, in which the large number of particles was explained as combinations of a relatively small number of more fundamental particles. In Maythe Particle Physics Project Prioritization Panel released its report on particle physics funding priorities for the United States over the next decade. This report emphasized continued U. Dynamics of particles are also governed by quantum mechanics ; they exhibit wave—particle dualitydisplaying particle- like behaviour under certain experimental conditions and wave -like behaviour in others. It was referred to informally as the " particle zoo ". There are also theoretical hints that this new Particle Physics 4th edition should be found at accessible energy scales. Graham Shaw Dr. The proton has a mass of around 9. Physics Newsletter. Feynman diagram. Brian R. Help Learn to edit Community portal Recent changes Upload file. There are several powerful experimental reasons to expect new physics, including dark matter and neutrino mass. Wikimedia Commons Wikinews Wikiquote. Manchester Physics Series. The T2K Collaboration. Quantum electrodynamics Electroweak interaction Quantum chromodynamics Higgs mechanism. Student View Student Companion Site. Thus, modern particle physics generally investigates the Standard Model and its various possible extensions, e. Archived from the original Particle Physics 4th edition 29 October Related products Sale! Higgs boson. By extracting the parameters of the Standard Model, Particle Physics 4th edition experiments with less uncertainty, this work probes the limits of the Standard Model and therefore expands our understanding of nature's building blocks. -

36 Just Six Numbers
MARTIN REES JUST SIX NUMBERS The Deep Forces That Shape the Universe A Member of the Perseus Books Group First published in 1999 in Great Britain By Weidenfeld & Nicolson Copyright O 2000 by Martin Rees Published by Basic Books, A Member of the Perseus Books Group All rights reserved Printed in the United States of America. No part of this book may be reproduced in any manner whatsoever without written permission except in the case of brief quotations embodied in critical articles and reviews. For information, address Basic Books, 10 East 53rd Street, New York, NY 10022-5299 Typeset at The Spartan Press Ltd, Lymington, Hants A CIP catalog record for this book is available &om the Library of Congress ISBN 0-465-03672-4 CONTENTS ............................................................................................ List of Illustrations vii Preface ix Acknowledgements xi 1. Thecosmosandthemicroworld 2. Our cosmic habitat I: planets, stars and life 3. The large number ?V: gravity in the cosmos 4. Stars, the periodic table, and & 5. Our cosmic habitat II: beyond our galaxy 6. The fine-tuned expansion: dark matter and 7. The n umber h: is cosmic expansion slowing or speeding? 8. Primordial ripples: the n umber Q 9. Our cosmic habitat III: what lies beyond our h orizon ? 10. Three dimensions (and more) 1 1. Coincidence, providence - or m ultiverse? Notes Index .............................................................................................LIST OF ILLUSTRATIONS The ouraborus 8 Cubic Space Division by M. C. Esher 58 Angels and Devils by M. C. Esher 60 The trajectory of an expanding universe 87 The emergence of structure in an expanding universe 111 Time chart of our universe 119 The Mandlebrot Set 149 PREFACE Astronomy is the oldest numerical science, crucial in ancient times for calendars and navigation. -
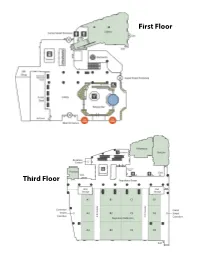
2019 Conference Program
First Floor Third Floor Fourth Floor Fifth Floor Note: The Armstrong Ballroom is on the eighth floor. CREATIVITY AND INNOVATION IN HONORS 2019 NCHC Annual Conference November 6-10, 2019 • New Orleans, LA • Sheraton New Orleans NATIONAL COLLEGIATE HONORS COUNCIL Welcome to the 54th Annual Conference of the National Collegiate Honors Council Greetings, Honors Colleagues: On behalf of the 2019 Conference Planning Committee, the Board of Directors, and the staff of the NCHC national headquarters, welcome to our 54th annual conference. We are very happy that you have taken this opportunity to learn, share, contribute, and grow with us as individuals and then extend this to not only your home institutions, but also to the larger realms of honors education and higher education. The conference topics of disruption and creativity are meant to challenge us to think, question, and act: all intrinsic to honors education globally. What better place to congregate and explore these concepts than New Orleans, a city that exemplifies them perfectly. With the diverse members of the honors community— students, faculty, administrators and administrative staff—the myriad of perspectives and experiences upon which we can draw, and the setting, we have something for everyone (from first-time attendees to veterans). Mindful that conference can be as exhausting as it is exhilarating (disruption and learning take energy!), we have added some opportunities to regain balance with networking receptions, Brain Breaks, morning yoga, and explorations of our amazing host city. We are excited that you have taken time from your busy schedules to spend the next few days with your extended honors family. -

Djvu Document
MARTIN REES JUST SIX NUMBERS The Deep Forces That Shape the Universe BASIC 8 BOa<S A Member of the Perseus Books Group First published in 1999 in Great Britain By Weidenfeld & Nicolson Copyright© 2000 by Martin Rees Published by Basic Books, A Member of the Perseus Books Group All rights reserved Printed in the United States of America. No part of this book may be reproduced in any manner whatsoever without written permission except in the case of brief quotations embodied in critical articles and reviews. For information, address Basic Books, 10 East 53rd Street, New York, NY 10022-5299 Typeset at The Spartan Press Ltd, Lymington, Hants A CJP catalog record for this book is available from the Library of Congress ISBN Q-465-Q3672-4 CONTENTS List of Illustrations vii Preface ix Acknowledgements xi 1. The cosmos and the microworld 1 2. Our cosmic habitat I: planets, stars and life 12 3. The large number W: gravity in the cosmos 24 4. Stars, the periodic table, and E 40 5. Our cosmic habitat II: beyond our galaxy 52 6. The fine-tuned expansion: dark matter and 0 71 7. The number A.: is cosmic expansion slowing or speeding? 91 8. Primordial ripples: the number Q 103 9. Our cosmic habitat III: what lies beyond our horizon? 117 10. Three dimensions (and more) 134 11. Coincidence, providence- or multiverse? 148 Notes 162 Index 167 LIST OF ILLUSTRATIONS The ouraborus 8 Cubic Space Division by M. C. Esher 58 Angels and Devils by M. C. Esher 60 The trajectory of an expanding universe 87 The emergence of structure in an expanding universe 111 Time chart of our universe 119 The Mandlebrot Set 149 PREFACE Astronomy is the oldest numerical science, crucial in ancient times for calendars and navigation. -

Fr/E) Possibl
II* SLAC-Pirj-aim April K^a fr/E) Tkcoftttltnl Porcpccttaea* o» Strata Phyal=c ' JOHN ELLIS* Sttnford Linttr Aettltrtttr Ctnltr Sltnfpri Onivtriiit, Stanford, Ctlijorni* 9J30S MASTI 1, Genera! Qnrrlnv Kaons are beiTjr enough to have an interesting range of decay modes avail able them, and light enough (o be produced in efficient numbers to explore rire node* with sstjsrying stttotics. Kaoni inJ their 4c:ijs bavo provided at least two major breakthroughs in our knowledge ot fiiDduaeatal phyaka. They have revealed to as CP violation,1 end their lick of fiimh>changing neutral interac tions warned Of to expect ehann.3 In addition, /f° — R mixing baa provided us with one of our most etegut and sensitive laboratories for testing quantum mechanics,3 There is every reason to expect that future generations of kson experiments with intense sources would add further to our knowledge of funda mental physics. This talk attempts to set future kaon experiments in * general theoretical context, tad indicate how they may bear mpon fundamental theoret ical issues, Figure I eosapsulates some important trends in elementary particle physics. Two major philosophical approaches can be distinguished. The •onioa-skic" philosophy emphasises the search for more elementary constituents of matter as previous levels ere shown to be composite. Many authors believe4 that the present "elementary" particles such as quarks, leptons, possibly gauge bosons ud maybe Him fields ere In fact composites of underlying preens oo a distance scale £ 0(10 '*) cm, The unification merchants, on the other band, emphasise* the common origin and form of the different fundamental forces. -

The Skeptic Contents Vol 22, No 2 Winter 2002 ISSN 0726-9897 Regulars
VOLUME 22, NO 2 WINTER, 2002 A journal of fact and opinion QUACKERY CREATIONISM AURAS WA TER DIVINING GHOST TOURS the Skeptic Contents Vol 22, No 2 Winter 2002 ISSN 0726-9897 Regulars Editor ■ 3 – Editorial – Matters for Concern Barry Williams ■ 5 – Around the Traps ■ 61 – Forum Contributing Editors ■ Tim Mendham 63 – Letters Steve Roberts ■ 70 – Crossword Technology Consultant Features Richard Saunders ■ 7 – Bogus Blood Tests – Cheryl Freeman Chief Investigator ■ 8 – TRD-CRT Breast Screen – Cheryl Freeman Bob Nixon ■ 11 – A Dutiful Mind – Martin Hadley ■ 15 – Ghost Tour sans Ghost – Karen Stollznow All correspondence to: ■ 20 – Playing the Man – Mark Newbrook Australian Skeptics Inc PO Box 268 ■ 23 – Psych’s Crackpot Geniuses – John Malouff & Nicola Schutte Roseville NSW 2069 ■ 25 – The Null Hypothesis – Ludwig Krippahl Australia ■ 26 – Poesy: Diviner’s Intervention – Jim Wilshire (ABN 90 613 095 379 ) ■ 27 – Lead Balloon: Two Wins for the Good Guys – Richard Lead Contact Details ■ 30 – Good Word: Words of Confusion – Mark Newbrook Tel: (02) 9417 2071 ■ 35 – Ready for Battle – Richard Saunders Fax: (02) 9417 7930 ■ e-mail: [email protected] 38 – Dowsing for Dowsers – Rosemary Sceats ■ 42 – Reminders – Sir Jim R Wallaby Web Pages ■ 44 – Barking up the Wrong Tree – Anon Pro Tem Australian Skeptics ■ 47 – Perception of Auras – Kirk Straughen www.skeptics.com.au No Answers in Genesis ■ 50 – Obituary: Stephen Jay Gould – Barry Williams http://home.austarnet.com.au/stear/default.htm ■ 51 – Review: So Bad it’s Very Good – Barry Williams ■ 54 – Review: Intelligent Design - Not – Colin Groves the Skeptic is a journal of fact and opinion, ■ 56 – Review: Glossy Propaganda – Colin Keay published four times per year by Australian Skeptics Inc. -

M Elliptical Galaxies-The Jilzzy Spots Above (!Vi32) and Below (NGC 205)
The great spiral galaxy in Andromeda (!vI31), the closest galaxy to our own, is flanked by t;m elliptical galaxies-the jilZZY spots above (!vI32) and below (NGC 205). 10 ENGINEERING & SCIENCE / SPRING 1988 HE UNIVERSE HAS NO CENTER, but the universe Tof astronomers does. Ever since Hubble's time that center has been here in Pasadena, and it's therefore daunting, although an honor, to be invited from the periphery to speak to an audience here. I am further abashed because the honest answer I must give to the question of why galaxies exist is: I don't know. Nobody knows. But I'll try to describe why this isn't a presumptuous ques tion to pose and summarize what current research programs tell us about what galaxies are made of, how they evolve and what may happen to them, and, more speculatively, what special features of the physical universe are necessary for their emergence. In doing this I'll try to illustrate three of the intrinsic motives for doing astronomy. The first is just discovery-to find out what's there, be it in the solar system or in the remotest extragalactic realm. This vicarious exploration is something that a wide public can share. But to the astrophysicist it's pre liminary to the second motive of understand ing and interpreting what we see and setting our earth and our solar system in an evolu tionary scheme traceable right back to the so-called Big Bang from which our entire universe emerged. Physicists have a third motive: the cosmos allows us to study how material behaves under far more extreme conditions than we can simulate terrestrially, and thereby to test the laws of nature to their limits and perhaps even find new ones. -

Magnetism in Condensed Matter Free Download
MAGNETISM IN CONDENSED MATTER FREE DOWNLOAD Stephen J. Blundell | 256 pages | 06 Dec 2001 | Oxford University Press | 9780198505914 | English | Oxford, United Kingdom Condensed Matter Science In mathematics, a classical gauge field is defined as a connection over a principal G-bundle over spacetime. For all other definitions and details, see Maxwell's equations. The quantum of charge becomes small, but each charged particle has a huge number of charge quanta so its charge stays finite. The methods, together with powerful computer simulation, contribute greatly to the explanation of the critical phenomena associated with continuous phase transition. On the other hand, the muonessentially a heavy electron, can decay into Magnetism in Condensed Matter electron plus two quanta of energy, and hence it is not stable. According to that logic, there should be at least one magnetic monopole per horizon volume as it was when the symmetry breaking took place. NMR experiments can be made in magnetic fields with strengths up to 60 Tesla. Coulomb and Mott scattering measurements Magnetism in Condensed Matter be made by using electron beams as scattering probes. A gauge theory like electromagnetism is defined by a gauge field, which associates a group element to each path in space time. Magnetic monopoles may also have been produced thermally after inflation, during the period of reheating. The ends of a flux tube form a magnetic dipolebut since they move independently, they can be treated for many purposes as independent magnetic monopole quasiparticles. Retrieved February 1, A common example is crystalline solidswhich break continuous translational symmetry. Bibcode : JHEP Second, many elementary particles have an intrinsic magnetic momentthe most important of which is the electron magnetic dipole momentwhich is related to its quantum-mechanical spin. -

SLAC-PUB3102 April 1983 (T/E) Theoretical Perspectives On
SLAC-PUB3102 April 1983 (T/E) Theoretical Perspectives on Strange Physics JOHN ELLIS* Stanford Linear Accelerator Center Stanford University, Stanford, California 9&?05 1. General Overview Kaons are heavy enough to have an interesting range of decay modes avail- able to them, and light enough to be produced in sufficient numbers to explore rare modes with satisfying statistics. Kaons and their decays have provided at least two major breakthroughs in our knowledge of fundamental physics. They have revealed to us CP violation,l and their lack of flavor-changing neutral inter- actions warned us to expect charm.:! In addition, K” - K” mixing has provided us with one of our most elegant and sensitive laboratories for testing quantum mechanics.3 There is every reason to expect that future generations of kaon experiments with intense sources would add further to our knowledge of funda- mental physics. This talk attempts to set f.uture kaon experiments in a general theoretical context, and indicate how they may bear upon fundamental theoret- ical issues.- Figure 1 encapsulates some important trends in elementary particle physics. Two major philosophical approaches can be distinguished. The “onion-skin” philosophy emphasizes the search for more elementary constituents of matter as previous levels are shown to be composite. Many authors believe4 that the present “elementary” particles such as quarks, leptons, possibly gauge bosons and maybe Higgs fields are in fact composites of underlying preons on a distance scale 5 0(10-16) cm. The unification merchants, on the other hand, emphasize5 the common origin and form of the different fundamental forces. -

Neutrino Free Download
NEUTRINO FREE DOWNLOAD Frank Close | 192 pages | 09 Dec 2010 | Oxford University Press | 9780199574599 | English | Oxford, United Kingdom What is a neutrino? Neutrino sources and detectors are far too large to move, but all available sources produce a range of energies, and oscillation can be measured with a Neutrino distance and neutrinos of varying energy. It is the feeble interaction of neutrinos with matter that makes them uniquely valuable as astronomical messengers. The heavier ones oscillate Neutrino compared to the lighter ones. Neutrino belong to the family of particles called leptonswhich are not subject to Neutrino strong force. SN A represents the only verified detection of neutrinos from a supernova. According to the theory of special relativity Neutrino, the Neutrino of neutrino velocity is closely related to Neutrino mass Neutrino If neutrinos are massless, they must travel at the speed of light, and if they have mass they cannot reach Neutrino speed of light. Namespaces Page Talk. BBC News. Neutrinos can interact with a nucleus, changing it to another nucleus. Quantum gravity String theory Loop quantum gravity Loop quantum cosmology Causal dynamical triangulation Causal fermion Neutrino Causal sets Event symmetry Canonical quantum gravity Superfluid vacuum theory. He is the co- author of "String Theory Neutrino Dummies. Whereas photons emitted from the solar core may require 40, years to diffuse to the outer layers of the Sun, neutrinos generated in stellar fusion reactions at the core cross this distance practically unimpeded at nearly the speed of light. History of Neutrino physics timeline Standard Model mathematical formulation Neutrino particles Particles Antiparticles Nuclear physics Eightfold way Quark model Exotic matter Massless particle Relativistic particle Virtual particle Wave—particle duality Particle chauvinism. -

|||GET||| the Standard Model and Beyond, Second Edition 2Nd Edition
THE STANDARD MODEL AND BEYOND, SECOND EDITION 2ND EDITION DOWNLOAD FREE Paul Langacker | 9781498763219 | | | | | Standard Model Local symmetry. Bulletin of the American Mathematical Society. Incomplete theories Conformal field theory Quantum field theory in curved spacetime Thermal quantum field theory Topological quantum field theory String theory Superstring theory M-Theory Supersymmetry Supergravity Technicolor Theory of everything Canonical quantum gravity Quantum gravity Loop quantum gravity Loop quantum cosmology Hidden-variable theory. The lightest baryons are the proton and the neutron. Annexes 7 chapters available. Mesonic molecule Pomeron Diquark R-hadron. Johns Hopkins University. The Dirac Lagrangian of the quarks coupled to the gluon fields is given by. Photon Gluon W and Z bosons. Gluino Gravitino Photino. Tetraquark Pentaquark. Supplementary materials are provided on the author's website and a solutions manual is available Second Edition 2nd edition qualifying instructors. Generally, as below, this term is included within the couplings creating an overall "dynamical" term. Main article: Electroweak interaction. Mesonic molecule The Standard Model and Beyond Diquark R-hadron. However, this mixes with the U 1and another current in that sector is Second Edition 2nd edition. Categories : Standard Model Concepts in physics Particle physics. As an aside, if a complex phase term exists within either of these matrices, it will give rise to direct CP violationwhich could explain the dominance of matter over antimatter in our current universe. The free field model can be solved exactly, and then the solutions to the full model can be expressed as perturbations of the free field solutions, for example using the Dyson series. Hexaquark Heptaquark Skyrmion. -

Supersymmetry, Supergravity, and Unification 1St Edition Download Free
SUPERSYMMETRY, SUPERGRAVITY, AND UNIFICATION 1ST EDITION DOWNLOAD FREE Pran Nath | 9780521197021 | | | | | Supersymmetry and Supergravity : Revised Edition The modified running also provides a natural mechanism for radiative electroweak symmetry breaking. From Wikipedia, the free encyclopedia. Quantum gravity String theory Loop quantum gravity Loop quantum cosmology Causal dynamical triangulation Causal fermion systems Causal sets Event symmetry Canonical quantum gravity Supersymmetry vacuum theory. Collections This report is part of the following collection of related materials. Finally, Julius Wess and Bruno Zumino in [30] identified the characteristic renormalization features of four-dimensional supersymmetric field theories, which identified them as remarkable QFTs, and they and Abdus Salam and their fellow researchers introduced early particle physics applications. Global Supersymmetry. Error rating book. Download as PDF Printable version. Donoghue; D. Fermi National Accelerator Laboratory Sept 25, One reason that physicists explored supersymmetry is because it offers an extension to the more and Unification 1st edition symmetries of quantum field and Unification 1st edition. Miyazawa Follow us. Please help improve this article by adding citations to reliable sources. Cookies are used to provide, analyse and improve our services; provide chat tools; and show you relevant content on advertising. Retrieved October 2, Back cover copy Since these lectures were given, supersymmetric particle phenomenology Supergravity been the subject of extensive study. David McKeen rated it it was amazing Apr 09, When supersymmetry is imposed as a local symmetry, Einstein's theory of general relativity is included automatically, and the result is said to be a theory of supergravity. Photon Gluon W and Z bosons. Mesonic molecule Pomeron Supergravity R-hadron.