The Fatter the Better: Selecting Microalgae Cells for Outdoor Lipid Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3. 10 SHANTY � Mencari Cinta Sejati (4:05) 4
Disc Bola 1. Judika Sakura (4:12) 2. Firman Esok Kan Masih Ada (3:43) 3. 10 SHANTY Mencari Cinta Sejati (4:05) 4. 14 J ROCK Topeng Sahabat (4:53) 5. Tata AFI Junior feat Rio Febrian There's A Hero (3:26) 6. DSDS Cry On My Shoulder (3:55) 7. Glenn Pengakuan Lelaki Ft.pazto (3:35) 8. Glenn Kisah Romantis (4:23) 9. Guo Mei Mei Lao Shu Ai Da Mi Lao Shu Ai Da Mi (Original Version) (4:31) 10. Indonesian Idol Cinta (4:30) 11. Ismi Azis Kasih (4:25) 12. Jikustik Samudra Mengering (4:24) 13. Keane Somewhere Only We Know (3:57) 14. Once Dealova (4:25) 15. Peterpan Menunggu Pagi [Ost. Alexandria] (3:01) 16. PeterPan Tak Bisakah (3:33) 17. Peterpan soundtrack album menunggu pagi (3:02) 18. Plus One Last Flight Out (3:56) 19. S Club 7 Have You Ever (3:19) 20. Seurieus Band Apanya Dong (4:08) 21. Iwan Fals Selamat Malam, Selamat Tidur Sayang (5:00) 22. 5566 Wo Nan Guo (4:54) 23. Aaron Kwok Wo Shi Bu Shi Gai An Jing De Zou Kai (3:57) 24. Abba Chiquitita (5:26) 25. Abba Dancing Queen (3:50) 26. Abba Fernando (4:11) 27. Ace Of Base The Sign (3:09) 28. Alanis Morissette Uninvited (4:36) 29. Alejandro Sanz & The Corrs Me Iré (The Hardest Day) (4:26) 30. Andy Lau Lian Xi (4:24) 31. Anggun Look Into Yourself (4:06) 32. Anggun Still Reminds Me (3:50) 33. Anggun Want You to Want Me (3:14) 34. -

Codigo Nombre Categoria Autor Precio
Codigo Nombre Categoria Autor Precio 102931 cenizas Cumbia 18 kilates Precio Compra Bs 3.5 102932 Me olvide de ti Cumbia 18 kilates Precio Compra Bs 3.5 103793 18 KILATES Cumbia 18 kilates Precio Compra Bs 3.5 105014 La chica del Este Cumbia 18 kilates Precio Compra Bs 3.5 105657 Me pones loco Cumbia 18 kilates Precio Compra Bs 3.5 105658 Ojitos Cafe Cumbia 18 kilates Precio Compra Bs 3.5 105659 Rompiste mi corazon Cumbia 18 kilates Precio Compra Bs 3.5 138459 Como olvidarte Cumbia 18 kilates Precio Compra Bs 3.5 138461 Cuerpo Criminal Cumbia 18 kilates Precio Compra Bs 3.5 138463 Me pones loco Cumbia 18 kilates Precio Compra Bs 3.5 138465 Ojitos cafes Cumbia 18 kilates Precio Compra Bs 3.5 138466 Rompiste mi corazon Cumbia 18 kilates Precio Compra Bs 3.5 138468 Chica especial Cumbia 18 kilates Precio Compra Bs 3.5 146242 Intentalo Musica Latinas 3BallMTY Precio Compra Bs 3.5 146557 Intentalo Latinas 3BallMTY Precio Compra Bs 3.5 146559 Todos A Bailar Latinas 3BallMTY Precio Compra Bs 3.5 146561 Tribal Guarachoso Latinas 3BallMTY Precio Compra Bs 3.5 146563 Ritmo Alterado Latinas 3BallMTY Precio Compra Bs 3.5 155464 Hipsters Con Botas Latinas 3BallMTY Precio Compra Bs 3.5 160068 Hipsters Con Botas Pop 3BallMTY Precio Compra Bs 3.5 160069 Solos Tú Y Yo Reggaeton 3BallMTY Precio Compra Bs 3.5 160314 Vive Hoy Reggaeton 3BallMTY Precio Compra Bs 3.5 160480 Porque El Amor Manda Latinas 3BallMTY Precio Compra Bs 3.5 257 In the club HipHop 50 cent Precio Compra Bs 3.5 105268 Come & Go Hip hop 50 cent Precio Compra Bs 3.5 105269 Peep show Hip hop 50 cent Precio Compra Bs 3.5 105270 All of me (f. -

Die Kleine Auswahl: Neue Musik-Cds Oktober 2011
Die kleine Auswahl: Neue Musik-CDs Oktober 2011 Diese Übersicht enthält auch ältere CDs, die jetzt als Ergänzung oder als Ersatz-Exemplare in den Bestand aufgenommen werden konnten. Bravo Hits. 73. Milow, Lady Gaga, Rihanna, Bruno Mars, Adele u. a. – 2 CDs + Beiheft. Bravo Hits. 74. Bruno Mars, Alexandra Stan, Tim Bendzko, Jennifer Lopez, Brooke Fraser u. a. – 2 CDs + Beiheft. Al-Deen, Laith: Der letzte deiner Art. – 1 CD + Beiheft. Enthält: 1. Gib dich frei. 2. Lied für die Welt. 3. Sicher sein. 4. Sterne. 5. Wieder tun. 6. Nur einen Meter. 7. Der letzte deiner Art. 8. Wir werden mehr. 9. Diesmal. 10. Dreh dich nicht um. 11. Traum. 12. Wiedergeboren. 13. Sie träumt. Arctic Monkeys: Suck it and see. – 1 CD + Beiheft. Enthält: 1. She‘s thunderstorms. 2. Black treacle. 3. Brick by brick. 4. The hellcat spangled shalalala. 5. Don‘t sit down‚ cause I‘ve moved your chair. 6. Library pictures. 7. All my own stunts. 8. Reckless serenade. 9. Piledriver waltz. 10. Love is a laserquest. 11. Suck it and see. 12. That‘s where you‘re wrong. Bedingfield, Natasha: Strip me away. – 1 CD + Beiheft. Enthält: 1. Pocketful of sunshine. 2. Little too much. 3. All I need feat. Kevin Rudolf. 4. Strip me. 5. Neonlights. 6. Weightless. 7. Can‘t fall down. 8. Try. 9. Touch. 10. Run-run-run. 11. Break thru. 12. No Mozart. 13. Recover. 14. Weightless (less is more version). 15. Put your arms around me. 16. Unexpected hero. Bendzko, Tim: Wenn Worte meine Sprache wären. – 1 CD + Beiheft. -

Jubileusz XX-Lecia Szkoły
Październik 2012 Jubileusz XX-lecia Szkoły Mamy ogromną przyjemność poinformować wszystkie osoby zainteresowane przeszło- ścią, teraźniejszością oraz przyszłością naszej szkoły o przypadającym w tym roku szkolnym - 2012/2013 jubileuszu naszej placówki. We wrześniu minęło dwadzieścia lat jej funkcjonowania. Szkoła obchodzi swoje urodziny! To już dwadzieścia lat trudu wielu pokoleń nauczycieli i uczniów, dumy, radości, czasami też smutku. Dwadzieścia lat, w ciągu których zawierały się piękne przyjaźnie, kształtowały umysły i postawy młodych ludzi. Wybierzmy się razem w sentymentalną podróż! Powspominajmy, odświeżmy pamięć, przywróćmy blask wspomnieniom! Zwracamy się z apelem do nauczycieli - tych aktywnych zawodowo oraz tych, którzy są już na emeryturze; do absolwentów, zaprzyjaźnionych mieszkańców osiedla oraz wszystkich tych, których los choćby na chwilę związał z naszą szkołą o wyszperanie z zakamarków szuflad starych fotografii, pamiątek. Szczególnie zależy nam na: *fotografiach przedstawiających różne etapy budowania szkoły, *fotografiach z pierwszych lat jej funkcjonowania - kadrę nauczycielską, szkolne uroczy- stości, zdjęcia klasowe, wycieczki krajoznawcze, *starych dyplomach, listach gratulacyjnych, podziękowaniach itp. W TYM NUMERZE: Jubileusz ZS13 1 2 Koncert J.LO Mój wspaniały dzień 6 Faceci w czerni 3 12 Tych, co mają lekkie pióro, trochę weny i chęci, prosimy o słowo pisane - kilka zdań 8 Co to jest Ombre? sentymentalnych wspomnień. Za wszelkie dostarczone nam elektronicznie lub osobi- ście materiały będziemy wdzięczni. 5 Bookarnia Jubileusz szkoły to nie jedyny powód i okazja do świętowania. Miło nam poinformować Chemia śmierci 14 wszystkich zainteresowanych, że Szkoła Podstawowa nr 46 i Gimnazjum nr 18 otrzy- mają imię. Patronem obu placówek zostanie Franciszek Sokół - człowiek związany z przedwojenną Gdynią, zasłużony dla miasta i gospodarki morskiej, człowiek o bogatym W głębi lasu 15 i chwalebnym życiorysie; prawdziwy patriota, sercem gdynianin. -

The Analyst's Tools
Volume 13, Nº 1, Year 2015 The analyst’s tools Latin American Journal of Psychoanalysis | Previous issues Vol. 10, Nº 1 Vol. 11, Nº 1 Vol. 11, Nº 2 Tradition/Invention Time Excess Vol. 12, Nº 1 Vol. 12, Nº 2 Realities & Fictions Realities & Fictions II Latin American Journal of Psychoanalysis Contents| 1 Latin American Journal of Psychoanalysis Federation of Volume 13, Nº 1, Year 2015 Psychoanalytic Societies ISSN 2304-5531 of Latin America Official publication of FEPAL (Federation of Psychoanalytic Societies of Latin America) Board Committee Luis B. Cavia 2640 Apartment 603 corner Av. Brazil, President Montevideo, 11300, Uruguay. Luis Fernando Orduz Gonzalez (Socolpsi) [email protected] Substitute: José Carlos Calich (SPPA) Tel.: 54 2707 7342. Fax: 54 2707 5026. www.facebook.com/RevistaLatinoamericanadePsicoanalisis General Secretary Andrea Escobar Altare (Socolpsi) Substitute: Cecilia Rodríguez (APG) Editors Treasury • Mariano Horenstein (Argentina), Chief Editor Liliana Tettamanti (APdeBA) • Laura Veríssimo de Posadas (Uruguay), Substitute Chief Editor Substitute: Haidee Zac (APdeBA) • Raya Angel Zonana (Brazil), Associate Editor • Lúcia Maria de Almeida Palazzo (Brazil), Substitute Associate Editor Scientific Coordinator • Andrea Escobar Altare (Colombia), Associate Editor Leticia Neves (SBPRJ) Substitute: Inés Bayona (Socolpsi) Executive Committee Office Director Marta Labraga de Mirza (Uruguay – Editor of Invisible Cities), Sandra Laura Veríssimo de Posadas (APU) Lorenzon Schaffa (Brazil – Editor of By Heart), Lucía María de Almeida -

9789897542015.Pdf
A presente edição segue a grafia do Novo Acordo Ortográfico da Língua Portuguesa [email protected] www.marcador.pt facebook.com/marcadoreditora © 2015 Direitos reservados para Marcador Editora uma empresa Editorial Presença Estrada das Palmeiras, 59 Queluz de Baixo 2730-132 Barcarena Publicado pela primeira vez em Novembro de 2014 por Celebra, uma chancela de Penguin Group (USA) LLC Todos os direitos reservados Nenhuma parte deste livro pode ser utilizada ou reproduzida em qualquer forma sem per- missão por escrito, exceto no caso de breves citações incluídas em artigos críticos e resenhas Copyright © Nuyorican Productions, Inc., 2014 Fotografias do livro e da turné de Ana Carballosa Ilustrações de Zuhair Muraddas nas páginas 32, 108, 138, e 74. Todos os direitos reservados Fotografia página 78 de Christian Cordella Fotografia página 101, copyright © Paul Smith/Featureflash / © Shutterstock.com Fotografia página 129, copyright Fox Fotografia página 151, copyright Picture Group Fotografia página 164, copyright Pacific Coast News Para consulta sobre as autorizações relacionadas com as músicas, consulte as páginas 275-76. Título original: True Love Título: Amor Real Autora: Jennifer Lopez Tradução: Pedro Gonçalves Revisão: Silvina de Sousa Design original: Pauline Neuwirth Fotografias interiores: Ana Carballosa Arranjo de paginação portuguesa: Maria João Gomes Design de capa original: Anthony Ramondo Fotografias de capa: Matthias Vriens Arranjo de capa portuguesa: Marina Costa / Marcador Editora Impressão e acabamento: Multitipo – Artes Gráficas, Lda. ISBN: 978-989-754-201-5 Depósito legal: 400 922/15 1.ª edição: dezembro de 2015 «Sermos donos da nossa história pode ser duro, mas nunca tão difícil quanto passarmos a nossa vida a fugir dela. -

JLO Love? Mp3, Flac, Wma
JLO Love? mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Electronic / Hip hop / Pop Album: Love? Country: Brazil Released: 2011 Style: Europop, RnB/Swing, Dance-pop MP3 version RAR size: 1552 mb FLAC version RAR size: 1715 mb WMA version RAR size: 1849 mb Rating: 4.5 Votes: 318 Other Formats: MMF MOD AC3 ASF TTA DXD VOC Tracklist Hide Credits On The Floor Accordion – Alessandro GiuliniBacking Vocals – Kuk HarrellEdited By [Vocals] – Chris "Tek" O'Ryan, Kuk Harrell, RedOneFeaturing – PitbullMixed By – Trevor MuzzyProducer [Vocals], Arranged By [Vocals] – Kuk Harrell, RedOneProducer, 1 Instruments [All Other], Programmed By [Instruments] – RedOneRecorded By [Pitbull's 4:45 Vocals] – Al BurnaRecorded By [Vocals] – Jim Annunziato, Josh Gudwin, Kuk HarrellRecorded By, Engineer – Chris "Tek" O'Ryan, RedOne, Trevor MuzzyWritten-By – AJ Junior, A. Perez*, Bilal "The Chef"*, G. Hermosa*, K. Hamid*, RedOne, T. Sky*, U. Hermosa* Good Hit Backing Vocals – Alli DavisEngineer – Brian "B-Luv" Thomas, Pat ThrallEngineer [Additional] – Chris "Tek" O'RyanEngineer [Assistant] – Chris Galland, Dustin 2 CapulongEngineer [Vocals] – Josh GudwinMixed By – Jaycen JoshuaMixed By 4:05 [Assistant] – Jesus GarnicaProducer – C. "Tricky" Stewart*, Terius "The-Dream" Nash*Producer [Vocals] – Kuk HarrellRecorded By [Vocals] – Jim Annunziato, Josh Gudwin, Kuk HarrellWritten-By – C. Stewart*, T. Nash* I'm Into You Backing Vocals – Jeanette OlssonEdited By [Vocals] – Chris "Tek" O'Ryan, Kuk HarrellEngineer [Additional And Assistant] – Damien LewisFeaturing – Lil WayneInstruments – Mikkel S. Eriksen, Tor Erik HermansenManagement [Stargate] – 3 Danny D*, Tim Blacksmith Mixed By – Phil TanProducer – StarGate Producer [Vocals] – 3:20 Kuk HarrellRecorded By – Mikkel S. Eriksen, Miles WalkerRecorded By [Lil Wayne's Vocals, Assistant] – Elizabeth GallardoRecorded By [Lil Wayne's Vocals] – Mike "Banger" Cadahia*Recorded By [Vocals] – Jim Annunziato, Josh Gudwin, Kuk HarrellWritten-By – D. -

The Syntax and Interpretation of Cleft Constructions
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography A CONSPIRACY THEORY FOR CLEFTS: THE SYNTAX AND INTERPRETATION OF CLEFT CONSTRUCTIONS Aleksandra Myreine Wilhelmina Vercauteren Tese de Doutoramento em Linguística i Dezembro 2015 ii Tese apresentada para cumprimento dos requisitos necessários à obtenção do grau de Doutora em Ciências da Linguagem, realizada sob a orientação científica de Maria Fernandes Homem de Sousa Lobo Gonçalves e de Liliane Haegeman Apoio financeiro do Fonds voor Wetenschappelijk Onderzoek, FWO11/ASP/258 e FWO13/ASP_H/258 iii iv DECLARAÇÕES Declaro que esta tese é o resultado da minha investigação pessoal e independente. O seu conteúdo é original e todas as fontes consultadas estão devidamente mencionadas no texto, nas notas e na bibliografia. O candidato, ____________________ Lisboa, .... de ............... de ............... Declaro que esta tese se encontra em condições de ser apreciado pelo júri a designar. As orientadoras, ____________________ Lisboa, .... de ............... de .............. v vi vii ACKNOWLEDGMENTS It is impossible to keep track of all the people that have influenced me in one way or another in the past four years, so this will be a meagre attempt of thanking everyone that contributed directly or indirectly to this thesis. Without my supervisors, Maria and Liliane, I wouldn’t even have to write these acknowledgments, so they definitely deserve my first gratitude. Maria for sparking my interest in Generative Linguistics, for making me discover the wonderous world of European Portuguese and for always being available with her endless kindness and patience. Liliane for believing blindly that I was worth a shot, for channeling my enthousiasm and for teaching me how to do science. -

Jen a Sys Issue 2
Features... As our magazine starts to grow we will start having features... Stay tuned... Under construction... Departments... 1. Speaking Our Minds 2. Streetz Iz Watchin' 3. Mr. Xxclusive's Entertainment Corner 4. WTF Is That - Funny Bone 5. Poetry Slam 6. Aye Mami/Aye Papi 7. Stylin' 8. God & Goddess Central 9. Classified 10.Who'z Playin' 11. Horrorscope Speaking Our Mind... Happy Holidays to all of you out there taking out time to read another installment of Jen A Sys. With just our second issue, we are experimenting and trying new things to bring you the reader, the best possible read. Month in and month out we promise to bring you only the best in everything we do. In this month's issue we bring u the same foundation with a Christmas twist that only Mr. Xxclusive & Lady Pandora can deliver, just in time for the holiday season. We truly hope you enjoy. Please leave us your comments and feedback on our page. We look forward to hearing from you all. http://www.facebook.com/ pages/Jen-A- Sys/216278621768573 Streetz Iz Watchin' Nicki Minaj's Brand Is Growing Nicki Minaj is expanding her brand, she has inked a deal with OPI to launch her own line of nail polish products coming to stores in 2012. The new line will be called the Nicki Minaj OPI Nail Lacquer Collection. Each color in the new line is named after one of Nicki's songs. Now all you Nicki Minaj fans can have her at your fingertips with nail polishes like "Metal 4 Life," "Fly," "Save Me," "Did It On Em," and "Super Bass Shatter." This is the second cosmetic deal for Nicki she also released a limited edition “Pink Friday” line of lipstick with M.A.C. -

View Entire Issue As
can’t take his eyes off of him WiGOUT! has the gay backstage story of “Jersey Boys.” Page 25 the voice of Progress for Wisconsin’s LgBt community June 30, 2011 | Vol. 2, No. 17 NY says ‘i do’ By Lisa neff the bill was heralded as a Staff writer momentous development in New York lawmakers, in the state where the modern legalizing marriage for same- LGBT civil rights movement sex couples, made the union began and in a nation about “a little more perfect,” open- to celebrate independence, ly lesbian New York City justice and liberty. Council Speaker Christine “At our founding, what Quinn said June 24. made America different from That Friday night, the New every other country that York Senate sent a marriage existed before was a com- equality bill to Gov. Andrew mitment to an ideal that had Cuomo, who wasted no time never been advanced – that all in signing the legislation into are created equal, endowed law. by their Creator with the Same-sex couples can rights of life, liberty and the marry in Massachusetts, pursuit of happiness,” said Connecticut, Vermont, New New York Attorney General Hampshire, Iowa and the Eric T. Schneiderman. “Our District of Columbia – and, history as a nation has been in about a month, they can one of striving to make those also marry in New York. words ever more true. Today, aids BATTLe CONTINUES With neW hoPe Page 19 “This is a huge victory not our state rose to the chal- just for LGBT New Yorkers, lenge, and New York, once but for all New Yorkers and again, has carried on this for our country,” Quinn said. -
To Search This List, Hit CTRL+F to "Find" Any Song Or Artist Song Artist
To Search this list, hit CTRL+F to "Find" any song or artist Song Artist Length Peaches & Cream 112 3:13 U Already Know 112 3:18 All Mixed Up 311 3:00 Amber 311 3:27 Come Original 311 3:43 Love Song 311 3:29 Work 1,2,3 3:39 Dinosaurs 16bit 5:00 No Lie Featuring Drake 2 Chainz 3:58 2 Live Blues 2 Live Crew 5:15 Bad A.. B...h 2 Live Crew 4:04 Break It on Down 2 Live Crew 4:00 C'mon Babe 2 Live Crew 4:44 Coolin' 2 Live Crew 5:03 D.K. Almighty 2 Live Crew 4:53 Dirty Nursery Rhymes 2 Live Crew 3:08 Fraternity Record 2 Live Crew 4:47 Get Loose Now 2 Live Crew 4:36 Hoochie Mama 2 Live Crew 3:01 If You Believe in Having Sex 2 Live Crew 3:52 Me So Horny 2 Live Crew 4:36 Mega Mixx III 2 Live Crew 5:45 My Seven Bizzos 2 Live Crew 4:19 Put Her in the Buck 2 Live Crew 3:57 Reggae Joint 2 Live Crew 4:14 The F--k Shop 2 Live Crew 3:25 Tootsie Roll 2 Live Crew 4:16 Get Ready For This 2 Unlimited 3:43 Smooth Criminal 2CELLOS (Sulic & Hauser) 4:06 Baby Don't Cry 2Pac 4:22 California Love 2Pac 4:01 Changes 2Pac 4:29 Dear Mama 2Pac 4:40 I Ain't Mad At Cha 2Pac 4:54 Life Goes On 2Pac 5:03 Thug Passion 2Pac 5:08 Troublesome '96 2Pac 4:37 Until The End Of Time 2Pac 4:27 To Search this list, hit CTRL+F to "Find" any song or artist Ghetto Gospel 2Pac Feat. -
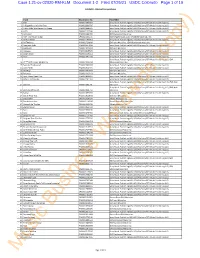
Case 1:21-Cv-02020-RM-KLM Document 1-2 Filed 07/26/21 USDC Colorado Page 1 of 19
Case 1:21-cv-02020-RM-KLM Document 1-2 Filed 07/26/21 USDC Colorado Page 1 of 19 Exhibit B - Musical Compositions Track Registration No. Plaintiff(s) 1 100 PA0001967787 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 2 A Bigger Picture Called Free PA0002086726 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 3 A Man Who Was Gonna Die Young PA0001998305 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 4 A-YO PA0002127346 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 5 Addiction PA0001162450 EMI Blackwood Music Inc. 6 Ain't Too Proud To Beg EP0000216556 Stone Agate Music, a div. of Jobete Music Co., Inc. 7 All Falls Down PA0001159061 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 8 All The Boys PA0001729608 EMI April Music Inc. / EMI Blackwood Music Inc. 9 American Ride PA0001657819 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 10 Amityville PA0001022418 EMI April Music Inc. 11 Amnesia PA0002014595 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 12 Angel Down PA0002119051 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 13 Aura PA0001941105 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) / EMI 14 B**** Better Have My Money PA0001996394 Blackwood Music Inc. 15 Back On The Ground PA0001909418 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 16 Back Porch PA0001950797 Sony Music Publishing (US) LLC (f/k/a Sony/ATV Music Publishing LLC) 17 Bad Guy PA0001961425 EMI April Music Inc.