Durham E-Theses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ON the ROCKS Newsletter of the Yorkshire Branch of the Open University Geological Society March 2018
ON THE ROCKS Newsletter of the Yorkshire Branch of the Open University Geological Society March 2018 A view of Great Gable (899m – the 9th highest mountain in England), Cumbria, looking northeast from the end of Wast Water, where the River Irt starts its short journey to the Irish Sea. Wast Water is the deepest lake in England (76m). The mountains are all from the Borrowdale Volcanic Group. (Peter Roberts 27.3.17 Grid Ref: NY 14535 03878) Welcome to the Spring edition of your newsletter Contents I hope you enjoy reading it and feel inspired to contribute to future issues. I must 1. Editor’s piece start with an apology. Unfortunately, the minutes of the AGM are not yet available 2. Rick’s musings but will be appearing in the next issue along with a copy of the accounts. 3. - 6. Blencathra report 7. Guide to minerals Our main article this time is the first of a number of reports by Peter Vallely on last 7. Obituary autumn’s Blencathra trip, and, if the photos are anything to go by, the hardy 8. Climate change article participants enjoyed a lovely sunny, if rather chilly, day out. 9. YOUGS 2018 field trips Peter Roberts has kindly provided the above photo, and we have another “simple 10. Snippets guide to minerals”, David Cousins’ personal view on surviving climate change, an 11. 2018 Blencathra obituary to Bill Graham who was a long-time Branch member, and a full listing of this year’s field trips, including separate details of this year’s Blencathra trip. -
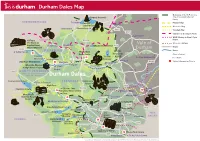
Durham Dales Map
Durham Dales Map Boundary of North Pennines A68 Area of Outstanding Natural Barleyhill Derwent Reservoir Newcastle Airport Beauty Shotley northumberland To Hexham Pennine Way Pow Hill BridgeConsett Country Park Weardale Way Blanchland Edmundbyers A692 Teesdale Way Castleside A691 Templetown C2C (Sea to Sea) Cycle Route Lanchester Muggleswick W2W (Walney to Wear) Cycle Killhope, C2C Cycle Route B6278 Route The North of Vale of Weardale Railway England Lead Allenheads Rookhope Waskerley Reservoir A68 Mining Museum Roads A689 HedleyhopeDurham Fell weardale Rivers To M6 Penrith The Durham North Nature Reserve Dales Centre Pennines Durham City Places of Interest Cowshill Weardale Way Tunstall AONB To A690 Durham City Place Names Wearhead Ireshopeburn Stanhope Reservoir Burnhope Reservoir Tow Law A690 Visitor Information Points Westgate Wolsingham Durham Weardale Museum Eastgate A689 Train S St. John’s Frosterley & High House Chapel Chapel Crook B6277 north pennines area of outstanding natural beauty Durham Dales Willington Fir Tree Langdon Beck Ettersgill Redford Cow Green Reservoir teesdale Hamsterley Forest in Teesdale Forest High Force A68 B6278 Hamsterley Cauldron Snout Gibson’s Cave BishopAuckland Teesdale Way NewbigginBowlees Visitor Centre Witton-le-Wear AucklandCastle Low Force Pennine Moor House Woodland ButterknowleWest Auckland Way National Nature Lynesack B6282 Reserve Eggleston Hall Evenwood Middleton-in-Teesdale Gardens Cockfield Fell Mickleton A688 W2W Cycle Route Grassholme Reservoir Raby Castle A68 Romaldkirk B6279 Grassholme Selset Reservoir Staindrop Ingleton tees Hannah’s The B6276 Hury Hury Reservoir Bowes Meadow Streatlam Headlam valley Cotherstone Museum cumbria North Balderhead Stainton RiverGainford Tees Lartington Stainmore Reservoir Blackton A67 Reservoir Barnard Castle Darlington A67 Egglestone Abbey Thorpe Farm Centre Bowes Castle A66 Greta Bridge To A1 Scotch Corner A688 Rokeby To Brough Contains Ordnance Survey Data © Crown copyright and database right 2015. -

Download Chapter In
Freshwater life Martyn G Kelly, Trevor D Cris, Ben Lamb and Brian Whitton Introduction Without the River Tees there would be no Teesdale and so, whilst much of the attention in this book is focussed on the plant and animal life in the fields and fells, we should not ignore either the river or the numerous tributary streams that feed it. Cow Green Reservoir, too, plays an important part in the story of Upper Teesdale, not just because of the ways in which it has altered the landscape and habitats in the upper valley, but also because the decision to impound the river precipitated many significant ecological studies and, ironically, raised the profile of the Teesdale rarities beyond a small band of botanical cognoscenti. The River Tees was the first British River to receive a detailed biological survey (by Butcher and colleagues in the mid-1930s). This was followed in the 1970s and 1980s by studies of the upper reaches of the main river and its tributaries by Durham University and the Freshwater Biological Association (later Institute of Freshwater Ecology and now Centre for Ecology and Hydrology). Since the previous edition of this book, further studies have investigated a wide range of factors including gravel, heavy metals, availability of salmonid spawning habitat and water colour. The upper tributaries of the Tees range from torrential streams, fed at times of peak flow mainly by surface run-off, to calcareous streams with some or much of their water from limestone springs. Those with the most water from springs are the ones which vary least in flow and have the highest calcium concentrations. -

Low Force and Holwick Are in the North Pennines Area of Outstanding Natural Beauty (AONB) and Geology and Landscape Around European Geopark
Low Force and Holwick are in the North Pennines Area of Outstanding Natural Beauty (AONB) and Geology and landscape around European Geopark European Geoparks The North Pennines AONB is Britain’s first European Low Force Geopark, a status supported by UNESCO, and a founding member of the Global Geoparks Network. Geoparks are special places with outstanding geology and landscape, and Holwick and where there are strong local efforts to make the most of geological heritage through interpretation, education, conservation and nature tourism. To find out more visit www.europeangeoparks.org A 2½-mile walk exploring landscape, Walk starts from here rocks, fossils and mines North Pennines Moor House – Upper Teesdale National Nature AONB & European Reserve (NNR) Geopark © Crown Copyright. All rights reserved. Part of this walk (south of the River Tees near Low Force) is Durham County Council. LA100049055. 2011. within the Moor House – Upper Teesdale NNR. This large reserve contains an almost complete range of upland For more information please contact: habitats typical of the North Pennines, from hay meadows North Pennines AONB Partnership, +44 (0)1388 528801 and juniper woods to limestone grassland and blanket bog. Weardale Business Centre, [email protected] It also includes the waterfalls of Cauldron Snout and High The Old Co-op Building, www.northpennines.org.uk 1 Martin Street, Stanhope, twitter.com/NorthPennAONB Force. For more information contact the Reserve Base on Bishop Auckland, County Durham facebook.com/NorthPenninesAONB 01833 622374. DL13 2UY Find out more about North Pennine geology This leaflet is one of a series of geological publications about the North Pennines. -

HADRIAN HUNDRED 25Th - 27Th MAY 2019
HADRIAN HUNDRED 25th - 27th MAY 2019 REGISTRATION – QUEEN ELIZABETH HIGH SCHOOL, HEXHAM NY 926 639 Welcome to Hexham once the haunt of marauding Vikings but now England’s favourite market town with the imposing Abbey at its hub. Starting in Northumberland the route visits Cumbria and Durham before returning to Northumberland for the later stages. Highlights include sections on Hadrian’s Wall, the South Tyne Trail, the Pennine Way (with Cross Fell and High Cup Nick), the Weardale Way and Isaac’s Tea Trail. Abbreviations TR Turn Right TL Turn Left N, S North, South etc. XXXm,Xkm Approx. distance in metres or kilometres to next feature (XXXdeg) Approx. magnetic bearing in degrees to next feature Units Convention Stage Summaries Miles & Kilometres (Distance), Feet & Metres (Ascent) Descriptive Text Metres (m) & Kilometres (km) NB. 100 metres = 109 yards 1 Kilometre = 0.62 miles Please note that all measurements of distance and ascent are produced from a GPS device which gives good estimates only therefore great accuracy cannot be guaranteed. 1 Important Notes A significant proportion of the Route uses or crosses roads, the vast majority of which are very minor and little used. The modern approach to Risk Assessment, however, requires that the risks involved in potentially mixing foot and vehicular traffic are pointed out whenever this happens. It is not proposed to mention this on every occasion that it occurs in the Route Description narrative. When a road is used or crossed the appropriate description will be highlighted. Additional warnings will be given whenever more major roads are encountered. Please be vigilant on roads especially later in the Event as you become increasingly tired and possibly less attentive. -

Moor House - Upper Teesdale B6278 Widdybank Farm, Langdon Beck, River Tees NNR Forest-In-Teesdale, B6277 Barnard Castle, Moor House – Cow Green Middleton- Co
To Alston For further information A686 about the Reserve contact: A689 The Senior Reserve Manager Moor House - Upper Teesdale B6278 Widdybank Farm, Langdon Beck, River Tees NNR Forest-in-Teesdale, B6277 Barnard Castle, Moor House – Cow Green Middleton- Co. Durham DL12 0HQ. Reservoir in-Teesdale To Penrith Tel 01833 622374 Upper Teesdale Appleby-in- National Nature Reserve Westmorland B6276 0 5km B6260 Brough To Barnard Castle B6259 A66 A685 c Crown copyright. All rights reserved. Kirkby Stephen Natural England 100046223 2009 How to get there Front cover photograph: Cauldron Snout The Reserve is situated in the heart of © Natural England / Anne Harbron the North Pennines Area of Outstanding Natural Beauty. It is in two parts on either Natural England is here to conserve and side of Cow Green Reservoir. enhance the natural environment, for its intrinsic value, the wellbeing and A limited bus service stops at Bowlees, enjoyment of people and the economic High Force and Cow Green on request. prosperity that it brings. There is no bus service to the Cumbria © Natural England 2009 side of the Reserve. ISBN 978-1-84754-115-1 Catalogue Code: NE146 For information on public transport www.naturalengland.org.uk phone the local Tourist Information Natural England publications are available Centres as accessible pdfs from: www.naturalengland.org.uk/publications Middleton-in-Teesdale: 01833 641001 Should an alternative format of this publication be required, please contact Alston: 01434 382244 our enquiries line for more information: 0845 600 3078 or email Appleby: 017683 51177 [email protected] Alston Road Garage [01833 640213] or Printed on Defra Silk comprising 75% Travel line [0870 6082608] can also help. -

THE SCENIC HIGHLIGHTS of the PENNINE WAY Discover the True Spirit of the North on England’S Original National Trail
ENGLAND’S GREAT WALKING TRAILS | THE PENNINE WAY LIMESTONE AND LEGEND: THE SCENIC HIGHLIGHTS OF THE PENNINE WAY Discover the true spirit of the North on England’s original National Trail Steeped in history and traversing spectacular upland landscapes in some of England’s most popular National ParKs, the Pennine Way is the most iconic of England’s Great WalKing Trails. Stretching for 268 miles (435Km) across England’s wild northern uplands, with a combined ascent that eXceeds the height of Mount Everest, it’s also arguably the toughest. OVERVIEW • Distance: 74 miles/120km • Start/Finish: Skipton/Appleby-in-Westmorland • Number of Days: 8 • Grade: Challenging • Theme: History / Geology • Landscape: Type High Hills & Moorland Opened in 1965, the Pennine Way blazed a trail for public access to some of England’s wildest landscapes – hitherto the sole preserve of a wealthy elite. Conceived by founder member of The Ramblers Tom Stephenson and popularised by the legendary Alfred Wainwright, the full route follows the rocky spine of England – stretching from the hills of the Derbyshire Peak District, through the Yorkshire Dales and onwards through Durham and Northumberland to the Scottish Border. Roughly following the line of the watershed from which great rivers like the Tyne and the Tees, the Lune and the Eden, flow east and west respectively, the bulk of this legendary trail lies at more than 1,000ft/305m above sea level. Our shortened 8-day itinerary explores the striking limestone landscapes of Malham in the Yorkshire Dales before climbing up onto the lonely massif of Cross Fell, where the Lakeland Fells are clearly visible across the Eden Valley. -

EFFECTS of COW GREEN RESERVOIR UPON DOWNSTREAM FISH POPULATIONS D. T. CRISP Introduction in 1967 the FBA Began Pre-Impoundment S
REPORT OF THE DIRECTOR 47 EFFECTS OF COW GREEN RESERVOIR UPON DOWNSTREAM FISH POPULATIONS D. T. CRISP Introduction In 1967 the FBA began pre-impoundment studies on fish populations at the site of the proposed Cow Green reservoir in upper Teesdale, (Crisp, Mann & McCormack 1974). Post-impoundment observations were made from 1971 to 1980 and some routine observations are still in progress. The project is concerned with fish populations in the River Tees downstream of the reservoir, in the reservoir itself and in the afferent streams, but the present account is confined to effects within the river downstream of the dam. A detailed account of physical and chemical effects was given by Crisp (1977) and a series of papers on effects upon invertebrates was reviewed by Armitage (1978a). The reservoir Cow Green reservoir is situated in the northern Pennines on the River Tees at Nat. Grid Ref. NY/813289 (Fig. 1). It has an area of 312 ha, a capacity of 40.9 m3x106, a top water level of 489 m O.D. and a maximum depth of 23 m. The catchment has an area of c. 5570 ha composed mainly of heather moor and blanket bog, but also containing areas of alluvial and limestone grassland. The function of the reservoir is river regulation - collection of water during periods of high river flow (late autumn to spring) and release of water during dry periods (chiefly summer and early autumn) - so as to maintain suitable river levels for downstream abstraction. The annual yield is 72 m3x106 yr_1, of which 14 m3x106 are released continuously as compensation flow (0.45 m3 s_1) and the remainder is released as required for river regulation. -

Petrology and Geochemistry of the Nipissing Gabbro: Exploration Strategies for Nickel, Copper, and Platinum Group Elements in a Large Igneous Province
Petrology and Geochemistry of the Nipissing Gabbro: Exploration Strategies for Nickel, Copper, and Platinum Group Elements in a Large Igneous Province Ontario Geological Survey Study 58 1996 Petrology and Geochemistry of the Nipissing Gabbro: Exploration Strategies for Nickel, Copper, and Platinum Group Elements in a Large Igneous Province Ontario Geological Survey Study 58 by P.C. Lightfoot and A.J. Naldrett 1996 Queen’s Printer for Ontario, 1996 ISSN 0704-2590 ISBN 0-778-4804-X All publications of the Ontario Geological Survey and the Ministry of Northern Development and Mines are available for viewing and purchase at the following locations: Mines and Minerals Information Centre (MMIC) Macdonald Block, Room M2-17 900 Bay Street Toronto, Ontario M7A 1C3 Telephone: 1-800-665-4480 (within Ontario) (416) 314-3800 Fax: (416) 314-3797 Publication Sales 933 Ramsey Lake Road, Level B2 Sudbury, Ontario P3E 6B5 Telephone: (705) 670-5691 Fax: (705) 670-5770 E-mail: [email protected] Use of Visa or Mastercard ensures the fastest possible service. Cheques or money orders should be made payable to the Minister of Finance. Canadian Cataloguing in Publication Data Lightfoot, Peter C. (Peter Charles) Petrology and Geochemistry of the Nipissing gabbro: exploration strategies for nickel, copper, and platinum group elements in a large igneous province (Ontario Geological Survey report, ISSN 0704-2590; 58) Includes bibliographical references. ISBN 0-7778-4804-X 1. Gabbro---Ontario---Nipissing Region. I. Naldrett, A.J. II. Ontario Ministry of Northern Development and Mines III. Ontario Geological Survey. IV. Series. QE462.G3L53 1995 552’.3 C95-964107-6 Every possible effort is made to ensure the accuracy of the information contained in this report, but the Ministry of Northern Development and Mines does not assume any liability for errors that may occur. -

Knock Geological Trail 3 Swindale Beck This Trail Is Approximately 9.5Km Long
the financial assistance of English Nature, Heritage Lottery Fund and the Countryside Agency Countryside the and Fund Lottery Heritage Nature, English of assistance financial the rdcdb ot ennsAN atesi,BiihGooia uvyadEgihNtr,with Nature, English and Survey Geological British Partnership, AONB Pennines North by Produced contact Appleby TIC: 017683 51177. 017683 TIC: Appleby contact For further information about the area, including public transport, public including area, the about information further For www.english-nature.org.uk Moor House-Upper Teesdale Reserve Base: 01833 622374 01833 Base: Reserve Teesdale House-Upper Moor English Nature Northumbria Team Office: 01661 845500 01661 Office: Team Northumbria Nature English Elizabeth Pickett (BGS) and to Eric Johnson Eric to and (BGS) Pickett Elizabeth Thanks to Charlotte Vye, Stu Clarke and Clarke Stu Vye, Charlotte to Thanks www.northpennines.org.uk Partnership on 01388 528801 or visit or 528801 01388 on Partnership contact the North Pennines AONB Pennines North the contact To find out more, out find To Network. Geoparks Please do not camp or light fires. light or camp not do Please founding member of the UNESCO Global UNESCO the of member founding also Britain's first European Geopark and a and Geopark European first Britain's also nesting birds and grazing livestock. grazing and birds nesting special places.The North Pennines AONB is AONB Pennines North places.The special keep dogs on a lead to avoid disturbing ground disturbing avoid to lead a on dogs keep , one of England's wildest and most and wildest England's of , one (AONB) Please follow the Countryside Code, in particular in Code, Countryside the follow Please Area of Outstanding Natural Beauty Natural Outstanding of Area this area. -

THE WHIN SILL Quartz-Dolerite, Dark in Colour
The rock itself is mainly fine to medium-grained THE WHIN SILL quartz-dolerite, dark in colour. It is tough stuff Alan Gill and makes excellent road stone. You may well have driven on it. One of the many interesting geological features in the north of England is the Whin Sill. This is essentially a subterranean layer of igneous rock underlying much of Northumberland, north east Cumbria, along the Pennine escarpment and in Teesdale. Its area is estimated to be at least 5000 sq km. The maximum thickness recorded is 75m but the average is between 25m and 50m. In many places the intrusion separates into two or more layers divided by several hundred metres induced by joint and fault planes. There are dykes associated with the Whin Sill forming together one single petrographic province. It is possible that these were the conduits through which the magma flowed prior to solidification. The proximity of the sill to the Carboniferous Limestone Series led some geologists to conclude that the sill originated as a contemporaneous lava flow. Sedgwick however advocated its Fig 2: High Force on the River Tees showing the intrusive origin as early as 1826, and was proved vertical structure of the Whin Sill resting on the right later in 1870. It was the subject, of pioneer horizontal Tynebottom Limestone. isotopic age determination by Arthur Holmes. The evidence suggests that the Whin Sill was intruded in late Carboniferous Times; an age of 295 +/- 6my has been computed. There are many good outcrops; the sea cliffs of the Farne Islands - the Romans saw a good thing when they built their wall along the north facing escarpments. -

THE TEESDALE WAY About the Author Martin Collins Is a Freelance Author, Photo-Journalist and Cartographer, As Well As a Regular Contributor to the UK Outdoor Media
THE TEESDALE WAY About the author Martin Collins is a freelance author, photo-journalist and cartographer, as well as a regular contributor to the UK outdoor media. First walking the GR5 in 1981 kindled a passion for the French Alps that remains as strong as ever. He has since written over twenty books for walkers embracing the coast, mountains and countryside of the UK and parts of Europe. He has three children, and lives in north Wales on the edge of the Snowdonia National Park. THE TEESDALE WAY by Martin Collins I’ve wandered many a weary mile, And in strange countries been; I’ve dwelt in towns and on wild moors, And curious sights I’ve seen; But still my heart clings to the dale Where Tees rolls to the sea; Compared with what I’ve seen I’ll say The Teesdale hills for me. (Richard Watson, the ‘Teesdale Poet’ – born Middleton-in-Teesdale 1833, died there 1891) JUNIPER HOUSE, MURLEY MOSS, OXENHOLME ROAD, KENDAL, CUMBRIA LA9 7RL www.cicerone.co.uk © Martin Collins 2005 CONTENTS Second edition 2005 ISBN: 978 1 85284 461 5 Overview map ...............................................................................................6–7 Reprinted 2013 (with updates) and 2019 Map key ............................................................................................................7 First edition 1995 Preface to the Second Edition ............................................................................8 Printed by KHL Printing, Singapore. Introduction ......................................................................................................9