Vibronic Assignments of Isomeric Trimethylbenzyl Radicals : Revisited†
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measuring and Predicting Sooting Tendencies of Oxygenates, Alkanes, Alkenes, Cycloalkanes, and Aromatics on a Unified Scale
Measuring and Predicting Sooting Tendencies of Oxygenates, Alkanes, Alkenes, Cycloalkanes, and Aromatics on a Unified Scale Dhrubajyoti D. Dasa,1, Peter St. Johnb,1, Charles S. McEnallya,∗, Seonah Kimb, Lisa D. Pfefferlea aYale University, Department of Chemical and Environmental Engineering, New Haven CT 06520 bNational Renewable Energy Laboratory, Golden CO 80401 Abstract Soot from internal combustion engines negatively affects health and climate. Soot emissions might be reduced through the expanded usage of appropriate biomass-derived fuels. Databases of sooting indices, based on measuring some aspect of sooting behavior in a standardized combustion environment, are useful in providing information on the comparative sooting tendencies of different fuels or pure compounds. However, newer biofuels have varied chemical structures including both aromatic and oxygenated functional groups, making an accurate measurement or prediction of their sooting tendency difficult. In this work, we propose a unified sooting tendency database for pure compounds, including both regular and oxygenated hydrocarbons, which is based on combining two disparate databases of yield-based sooting tendency measurements in the literature. Unification of the different databases was made possible by leveraging the greater dynamic range of the color ratio pyrometry soot diagnostic. This unified database contains a substantial number of pure compounds (≥ 400 total) from multiple categories of hydrocarbons important in modern fuels and establishes the sooting tendencies of aromatic and oxygenated hydrocarbons on the same numeric scale for the first time. Using this unified sooting tendency database, we have developed a predictive model for sooting behavior applicable to a broad range of hydrocarbons and oxygenated hydrocarbons. The model decomposes each compound into single-carbon fragments and assigns a sooting tendency contribution to each fragment based on regression against the unified database. -
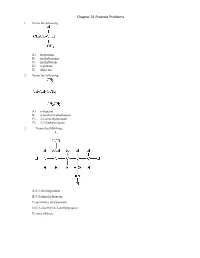
Chapter 21 Practice Problems 1
Chapter 21 Practice Problems 1. Name the following: A) isopropane B) methylpentane C) methylbutane D) n-pentane E) dodecane 2. Name the following: A) n-heptane B) 2-methyl-2-ethylbutane C) 3,3-dimethylpentane D) 2,2-diethylpropane 3. Name the following: A) 2,4-diethylpentane B) 3,5-dimethylheptane C) secondary ethylpentane D) 2,3-dimethyl-2,3-diethylpropane E) none of these 4. In lecture, a professor named a molecule 2-ethyl-4-tert-butylpentane. A student pointed out that the name was incorrect. What is the correct systematic name for the molecule? A) 2-t-butyl-5-methylhexane B) 2-ethyl-4,5,5-trimethylhexane C) 3,5,6,6-tetramethylheptane D) 2,2,3,5-tetramethylheptane E) undecane 5. Structural isomers have A) different molecular formulas and different structures. B) different molecular formulas but the same structure. C) the same molecular formula and the same structure. D) the same molecular formula but different structures. E) none of these 6. How many structural isomers does propane have? A) 3 B) 2 C) 1 D) 5 E) 4 7. The product of ethane undergoing dehydrogenation is called A) propene. B) methene. C) ethene. D) propane. E) none of these 8. Which of the following, upon reacting with oxygen, would form the greatest amount of carbon dioxide? A) n-pentane B) isopentane C) neopentane D) Two of these would form equal amounts. E) All of these would form equal amounts. 9. Which of the following has the lowest boiling point? A) methane B) butane C) ethane D) propane E) All of these have the same boiling point. -

Δ13c of Aromatic Compounds in Sediments, Oils And
1 δ13C of aromatic compounds in sediments, oils and 2 atmospheric emissions: a review 3 Alex I. Holman a, Kliti Grice a* 4 5 a Western Australia Organic and Isotope Geochemistry Centre, The Institute for 6 Geoscience Research, School of Earth and Planetary Sciences, Curtin University, 7 GPO Box U1987, Perth, WA 6845, Australia 8 9 * Corresponding author 10 Alex Holman: Tel.: +61(0) 8 9266 9723. E-mail address: [email protected] 11 Kliti Grice: Tel.: +61(0) 8 9266 2474. E-mail address: [email protected] 12 13 14 15 16 17 18 19 Abstract 20 This review discusses major applications of stable carbon isotopic 21 measurements of aromatic compounds, along with some specific technical aspects 22 including purification of aromatic fractions for baseline separation. δ13C 23 measurements of organic matter (OM) in sediments and oils are routine in all 24 fields of organic geochemistry, but they are predominantly done on saturated 25 compounds. Aromatic compounds are important contributors to sedimentary 26 organic matter, and provide indication of diagenetic processes, OM source, and 27 thermal maturity. Studies have found evidence for a small 13C-enrichment during 28 diagenetic aromatisation of approximately 1 to 2 ‰, but the formation of polycyclic 29 aromatic hydrocarbons (PAHs) from combustion and hydrothermal processes 30 seems to produce no effect. Likewise, maturation and biodegradation also produce 31 only small isotopic effects. An early application of δ13C of aromatic compounds was 32 in the classification of oil families by source. Bulk measurements have had some 33 success in differentiating marine and terrigenous oils, but were not accurate in all 34 settings. -

Effects of Sulfide Minerals on Aromatic Maturity Parameters
Organic Geochemistry 76 (2014) 270–277 Contents lists available at ScienceDirect Organic Geochemistry journal homepage: www.elsevier.com/locate/orggeochem Effects of sulfide minerals on aromatic maturity parameters: Laboratory investigation using micro-scale sealed vessel pyrolysis ⇑ ⇑ Alex I. Holman a, , Paul F. Greenwood a,b,c, Jochen J. Brocks d, Kliti Grice a, a Western Australia Organic and Isotope Geochemistry Centre, Department of Chemistry, The Institute for Geoscience Research, Curtin University, GPO Box U1987, Perth, WA 6845, Australia b Centre for Exploration Targeting, University of Western Australia, Crawley, WA 6009, Australia c WA Biogeochemistry Centre, University of Western Australia, Crawley, WA 6009, Australia d Research School of Earth Sciences, The Australian National University, Canberra, ACT 0200, Australia article info abstract Article history: Sedimentary organic matter from the Here’s Your Chance (HYC) Pb–Zn–Ag deposit (McArthur Basin, Received 19 December 2013 Northern Territory, Australia) displays increased thermal maturity compared to nearby non-mineralised Received in revised form 28 August 2014 sediments. Micro-scale sealed vessel pyrolysis (MSSVpy) of an immature, organic rich sediment from the Accepted 2 September 2014 host Barney Creek Formation (BCF) was used to simulate the thermal maturation of OM from the HYC Available online 16 September 2014 deposit, and to assess the effect of sulfide minerals on organic maturation processes. MSSVpy at increas- ing temperatures (300, 330 and 360 °C) resulted in increased methylphenanthrene maturity ratios which Keywords: were within the range reported for bitumen extracted from HYC sediments. The methylphenanthrene Micro-scale sealed vessel pyrolysis index ratio from MSSVpy of the BCF sample was lower than in HYC, due to a reduced proportion of meth- Polycyclic aromatic hydrocarbon Thermal maturity ylated phenanthrenes. -

1,2,4-Trimethylbenzene Transformation Reaction Compared with Its Transalkylation Reaction with Toluene Over USY-Zeolite Catalyst
1,2,4-Trimethylbenzene Transformation Reaction Compared with its Transalkylation Reaction with Toluene over USY-Zeolite Catalyst Sulaiman Al-Khattaf*, Nasir M. Tukur, and Adnan Al-Amer Chemical Engineering Department, King Fahd University of Petroleum & Minerals Dhahran 31261, Saudi Arabia Abstract 1,2,4-Trimethyl benzene (TMB) transalkylation with toluene has been studied over USY-zeolite type catalyst using a riser simulator that mimics the operation of a fluidized-bed reactor. 50:50 wt% reaction mixtures of TMB and toluene were used for the transalkylation reaction. The range of temperature investigated was 400-500 oC and time on stream ranging from 3 to 15 seconds. The effect of reaction conditions on the variation of p-xylene to o-xylene products ratio (P/O), distribution of trimethylbenzene (TMB) isomers (1,3,5-to-1,2,3-) and values of xylene/tetramethylbenzenes (X/TeMB) ratios are reported. Comparisons are made between the results of the transalkylation reaction with the results of pure 1,2,4-TMB and toluene reactions earlier reported. Toluene that was found almost inactive, became reactive upon blending with 1,2,4-TMB. This shows that toluene would rather accept a methyl group to transform to xylene than to loose a methyl group to form benzene under the present experimental condition with pressures around ambient. The experimental results were modeled using quasi-steady state approximation. Kinetic parameters for the 1,2,4-TMB disappearance during the transalkylation reaction, and in its conversion into isomerization and disproportionation products were calculated using the catalyst activity decay function based on time on stream (TOS). -
![United States Patent [19] [11] Patent Number: 6,147,270 Pazzucconi Et Al](https://docslib.b-cdn.net/cover/4104/united-states-patent-19-11-patent-number-6-147-270-pazzucconi-et-al-1394104.webp)
United States Patent [19] [11] Patent Number: 6,147,270 Pazzucconi Et Al
US006147270A United States Patent [19] [11] Patent Number: 6,147,270 Pazzucconi et al. [45] Date of Patent: Nov. 14, 2000 [54] PROCESS FOR THE PREPARATION OF 5,670,704 9/1997 Hagen et al. ......................... .. 585/471 2,6-DIMETHYLNAPHTHALENE USING A 5,672,799 9/1997 Perego et al. ......................... .. 585/467 MTW ZEOLITIC CATALYST FOREIGN PATENT DOCUMENTS [75] Inventors: Giannino Pazzucconi, Broni; Carlo 2 246 788 2/1992 United Kingdom . Perego, Carnate; Roberto Millini, WO 90/03960 4/1990 WIPO. Cerro al Lambro; Francesco Frigerio, OTHER PUBLICATIONS Torre d’lsola; Riccardo Mansani, Sassari; Daniele Rancati, Porto Torres, “MTW”; internet search record, Dec. 1999. all of Italy Primary Examiner—Marian C. Knode [73] Assignee: Enichem S.p.A., S. Donato Milanese, Assistant Examiner—Thuan D. Dang Italy Attorney, Agent, or Firm—Oblon, Spivak, McClelland, Maier & Neustadt, PC. [21] Appl. No.: 09/281,961 [57] ABSTRACT [22] Filed: Mar. 31, 1999 A highly selective process is described for preparing 2,6 dimethylnaphthalene Which comprises reacting a naphtha [30] Foreign Application Priority Data lene hydrocarbon selected from naphthalene, Apr. 17, 1998 [IT] Italy ............................... .. MI98A0809 methylnaphthalenes, dimethylnaphthalenes, trimethylnaphthalenes, polymethylnaphthalenes or their [51] Int. Cl.7 ................................................... .. C07C 15/12 mixtures With one or more benzene hydrocarbons selected [52] US. Cl. ........................................... .. 585/475; 585/471 from benzene, toluene, Xylenes, trimethylbenZenes, [58] Field of Search ................................... .. 585/475, 471, tetramethylbenZenes, pentamethylbenZene and/or 585/470 heXamethylbenZene, under at least partially liquid phase conditions, in the presence of a Zeolite belonging to the [56] References Cited structural type MTW and optionally in the presence of a U.S. -

Disproportionation of 1,2,4-Trimethylbenzene Over Zeolite NU-87
Korean J. Chem. Eng., 17(2), 198-204 (2000) Disproportionation of 1,2,4-Trimethylbenzene over Zeolite NU-87 Se-Ho Park, Jong-Hyung Lee and Hyun-Ku Rhee† School of Chemical Engineering and Institute of Chemical Processes, Seoul National University, Kwanak-ku, Seoul 151-742, Korea (Received 27 September 1999 • accepted 30 December 1999) Abstract−The catalytic properties of zeolite NU-87 were investigated with respect to the disproportionation of 1, 2,4-trimethylbenzene and the results were compared to those obtained over H-beta and H-mordenite with 12-mem- bered ring channel system. In the conversion of 1,2,4-trimethylbenzene, the disproportionation to xylene and tetra- methylbenzene is preferred to the isomerization into 1,2,3- and 1,3,5-isomers over all the three zeolites, but this trend is much more pronounced over HNU-87 owing to its peculiar pore structure. Disproportionation reaction is found to proceed within the micropores of HNU-87, whereas isomerization occurs mainly on the external surface. The high selectivity to disproportionation gives more xylenes and tetramethylbenzenes over HNU-87. The detailed descrip- tions for the product distribution are also reported. Key words: NU-87, 1,2,4-Trimethylbenzene, Disproportionation, Isomerization INTRODUCTION EXPERIMENTAL Disproportionation of trimethylbenzene (TMB) to xylene and 1. Catalysts Preparation = tetramethylbenzene (TeMB) is an important process for the indus- H-mordenite (Engelhard, SiO2/Al2O3 45) and H-beta (PQ Corp., = try, mainly due to the increasing demand for p-xylene to be used SiO2/Al2O3 22) used in this study were taken from commercial for the production of polyester resins. -

Trimethylbenzoic Acids As Metabolite Signatures in the Biogeochemical Evolution of an Aquifer Contaminated with Jet Fuel Hydrocarbons
Journal of Contaminant Hydrology 67 (2003) 177–194 www.elsevier.com/locate/jconhyd Trimethylbenzoic acids as metabolite signatures in the biogeochemical evolution of an aquifer contaminated with jet fuel hydrocarbons J.A. Namocatcata,1, J. Fanga,*, M.J. Barcelonaa,2, A.T.O. Quibuyenb, T.A. Abrajano Jr.c a National Center for Integrated Bioremediation Research and Development, Department of Civil and Environmental Engineering, The University of Michigan, Ann Arbor, MI 48109, USA b Institute of Chemistry, University of the Philippines, Diliman, Quezon City 1101, Philippines c Department of Earth and Environmental Sciences, Rensselaer Polytechnic Institute, Troy, NY 12180, USA Received 24 May 2002; accepted 21 March 2003 Abstract Evolution of trimethylbenzoic acids in the KC-135 aquifer at the former Wurtsmith Air Force Base (WAFB), Oscoda, MI was examined to determine the functionality of trimethylbenzoic acids as key metabolite signatures in the biogeochemical evolution of an aquifer contaminated with JP-4 fuel hydrocarbons. Changes in the composition of trimethylbenzoic acids and the distribution and concentration profiles exhibited by 2,4,6- and 2,3,5-trimethylbenzoic acids temporally and between multilevel wells reflect processes indicative of an actively evolving contaminant plume. The concentration levels of trimethylbenzoic acids were 3–10 orders higher than their tetramethylben- zene precursors, a condition attributed to slow metabolite turnover under sulfidogenic conditions. The observed degradation of tetramethylbenzenes into trimethylbenzoic acids obviates the use of these alkylbenzenes as non-labile tracers for other degradable aromatic hydrocarbons, but provides rare field evidence on the range of high molecular weight alkylbenzenes and isomeric assemblages amenable to anaerobic degradation in situ. -

"Hydrocarbons," In: Ullmann's Encyclopedia of Industrial Chemistry
Article No : a13_227 Hydrocarbons KARL GRIESBAUM, Universit€at Karlsruhe (TH), Karlsruhe, Federal Republic of Germany ARNO BEHR, Henkel KGaA, Dusseldorf,€ Federal Republic of Germany DIETER BIEDENKAPP, BASF Aktiengesellschaft, Ludwigshafen, Federal Republic of Germany HEINZ-WERNER VOGES, Huls€ Aktiengesellschaft, Marl, Federal Republic of Germany DOROTHEA GARBE, Haarmann & Reimer GmbH, Holzminden, Federal Republic of Germany CHRISTIAN PAETZ, Bayer AG, Leverkusen, Federal Republic of Germany GERD COLLIN, Ruttgerswerke€ AG, Duisburg, Federal Republic of Germany DIETER MAYER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany HARTMUT Ho€KE, Ruttgerswerke€ AG, Castrop-Rauxel, Federal Republic of Germany 1. Saturated Hydrocarbons ............ 134 3.7. Cumene ......................... 163 1.1. Physical Properties ................ 134 3.8. Diisopropylbenzenes ............... 164 1.2. Chemical Properties ............... 134 3.9. Cymenes; C4- and C5-Alkylaromatic 1.3. Production ....................... 134 Compounds ...................... 165 1.3.1. From Natural Gas and Petroleum . .... 135 3.10. Monoalkylbenzenes with Alkyl Groups 1.3.2. From Coal and Coal-Derived Products . 138 >C10 ........................... 166 1.3.3. By Synthesis and by Conversion of other 3.11. Diphenylmethane .................. 167 Hydrocarbons . .................. 139 4. Biphenyls and Polyphenyls .......... 168 1.4. Uses ............................ 140 4.1. Biphenyl......................... 168 1.5. Individual Saturated Hydrocarbons ... 142 4.2. Terphenyls...................... -

Electrophilic Mercuration and Thallation of Benzene and Substituted Benzenes in Trifluoroacetic Acid Solution* (Electrophilic Substitution/Selectivity) GEORGE A
Proc. Natl. Acad. Sci. USA Vol. 74, No. 10, pp. 4121-4125, October 1977 Chemistry Electrophilic mercuration and thallation of benzene and substituted benzenes in trifluoroacetic acid solution* (electrophilic substitution/selectivity) GEORGE A. OLAH, IWAO HASHIMOTOt, AND HENRY C. LINtt Institute of Hydrocarbon Chemistry, Department of Chemistry, University of Southern California, Los Angeles, California 90007; and the Department of Chemistry, Case Western Reserve University, Cleveland, Ohio 44101 Contributed by George A. Olah, July 18, 1977 ABSTRACT The mercuration and thallation of benzene and iments at 250 and quenched the mixtures after 5 min, whereas substituted benzenes was studied with mercuric and thallic Brown and Nelson carried out the reaction for 6.5 hr. In sub- trifluoroacetate, respectively, in trifluoroacetic acid. With the in view of the importance of both the mechanistic shortest reaction time (1 sec) at 00, the relative rate of mercu- sequent work, ration of toluene compared to that of benzene was 17.5, with and practical implications of the orientation-rate correlation, the isomer distribution in toluene of: ortho, 17.4%; meta, 5.9%; Brown and McGary (7), carried out a more detailed study of and para, 76.7%. The isomer distribution in toluene varied with the mercuration reaction. They concluded: "A redetermination the reaction time, significantly more at 25° than at 00. The of the isomer distributions and relative rates indicates excellent competitive thallation of benzene and toluene with thallic tri- agreement with the linear relationship of orientation and rel- fluoroacetate in trifluoroacetic acid at 150 showed the relative ative however, that the isomer distri- rate, toluene/benzene, to be 33, with the isomer distribution in rate." They recognized, toluene of: ortho, 9.5%; meta, 5.5%; and para, 85.0%. -

145053318.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) STRATEGIES FOR THE IMPROVEMENT OF THE INDUSTRIAL OXIDATION OF CYMENE by NIGEL HARMSE Baccalareus Technologiae Chemistry (PET) A dissertation submitted for the fulfilment of the requirements for the Masters Degree in Technology: Chemistry in the Faculty of Applied Science at the Port Elizabeth Technikon January 2001 Promoter : Prof. B Zeelie II ACKNOWLEDGEMENTS ■ My promoter Prof. Ben Zeelie for his help and guidance. ■ To Dr. Barton for her assistance. ■ To Sasol for their financial assistance. ■ To my Heavenly Father for being my shield and strength. ■ My parents for their support. ■ Special thanks to my girlfriend Carmen, and my daughter Cassandra, for encouragement and inspiration during this write-up. ■ To Max and friends in PETCRU (Port Elizabeth Technikon Catalyst Research Unit) for their help. III CONTENTS ACKNOWLEDGEMENTS CONTENTS SUMMARY CHAPTER 1 INTRODUCTION 1.1 GENERAL 1 1.2 A BRIEF HISTORY OF THE SOUTH AFRICAN INDUSTRY 2 1.2.1. Current Trends 3 1.2.2. Coal Chemicals versus Oil Chemicals 5 1.3 OVERVIEW OF CRESOLS 9 1.3.1 Introduction 9 1.3.2 Properties 10 1.3.3 Market Trends 11 1.3.4 Uses and Applications 12 1.3.4.1 Agricultural chemicals 12 1.3.4.2 Antioxidants 12 1.3.4.3 Flavour and fragrances 12 1.3.4.4 Pharmaceuticals 13 1.3.5 Sources 13 1.3.5.1 Natural Sources 13 1.3.5.1.1 Coal Tar Acids 13 1.3.5.1.2 Refinery Spent Caustic 14 1.3.5.2 Synthetic Sources 14 1.3.5.2.1 Phenol Methylation 14 -
Aromatic 150 Technical Data
Technical Data: Aromatic 150 Product Description Atosol 150 Solvent naphtha (petroleum), heavy arom. Atosol 150 is a complex mixture of aromatic hydrocarbons which is predominately composed of C10–C12 aromatics in the form of alkylbenzenes and alkylnaphthalenes from distillation of petroleum. More specifically, it contains significant amounts of dimethyl-ethylbenzenes and tetramethylbenzenes. It also contains naphthalene (CAS RN 91-20-3) at levels typically less than 10 %. It contains less than 0.1 % ethylbenzene (CAS RN 100-41-4 ) and benzene (CAS RN 71-43 -2). This product may contain approximately 25 ppm BHT (CAS RN 128-37-0). Property Units Specifications Typical Test Method Appearance Colorless Liquid Color, Saybolt 30min +30 Specific Gravity @15.65/15.65˚C 0.89-0.91 0.9839 Distillation ˚C 179min 220max 182-207 Aromatics vol% 99.9 Olefins vol% <0.1 Saturates vol% <0.1 Sulfur mg/kg 5max <2 Flash Point (PMCC) ˚C 60min 64.0 Aniline Point ˚C 18 max 11 Kauri-Butanol Value (KB) 90min 95.6 Carolina International Sales Co., Inc. www.ciscochem.com Technical Data: Aromatic 150 Item Number Date Created Date Updated 5/20/2015 5/20/2015 CAROLINA INTERNATIONAL SALES CO., INC. 266 Rue Cezzan Lavonia, GA 30553 770-904-7042 www.ciscochem.com Limitations of Warranty & Liability Seller warrants its product will meet the specifications listed herein and it has good title to the product, free and clear of all liens and security interests; SELLER MAKES NO OTHER WARRANTY, WHETHER EXPRESSED OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Buyer or user accepts liability for determining if the product is suitable for Buyer’s or user’s intended use.