Chemical Quality of Base Flow in 18 Selected Streams in the Upper Susquehanna River Basin, New York
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5
NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5 DATABASE DESIGN DOCUMENTATION AND DATA DICTIONARY 1 June 2013 Prepared for: United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, Maryland 21403 Prepared By: Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 Prepared for United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, MD 21403 By Jacqueline Johnson Interstate Commission on the Potomac River Basin To receive additional copies of the report please call or write: The Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 301-984-1908 Funds to support the document The Non-Tidal Benthic Monitoring Database: Version 3.0; Database Design Documentation And Data Dictionary was supported by the US Environmental Protection Agency Grant CB- CBxxxxxxxxxx-x Disclaimer The opinion expressed are those of the authors and should not be construed as representing the U.S. Government, the US Environmental Protection Agency, the several states or the signatories or Commissioners to the Interstate Commission on the Potomac River Basin: Maryland, Pennsylvania, Virginia, West Virginia or the District of Columbia. ii The Non-Tidal Benthic Monitoring Database: Version 3.5 TABLE OF CONTENTS BACKGROUND ................................................................................................................................................. 3 INTRODUCTION .............................................................................................................................................. -

Susquehanna Riyer Drainage Basin
'M, General Hydrographic Water-Supply and Irrigation Paper No. 109 Series -j Investigations, 13 .N, Water Power, 9 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR HYDROGRAPHY OF THE SUSQUEHANNA RIYER DRAINAGE BASIN BY JOHN C. HOYT AND ROBERT H. ANDERSON WASHINGTON GOVERNMENT PRINTING OFFICE 1 9 0 5 CONTENTS. Page. Letter of transmittaL_.__.______.____.__..__.___._______.._.__..__..__... 7 Introduction......---..-.-..-.--.-.-----............_-........--._.----.- 9 Acknowledgments -..___.______.._.___.________________.____.___--_----.. 9 Description of drainage area......--..--..--.....-_....-....-....-....--.- 10 General features- -----_.____._.__..__._.___._..__-____.__-__---------- 10 Susquehanna River below West Branch ___...______-_--__.------_.--. 19 Susquehanna River above West Branch .............................. 21 West Branch ....................................................... 23 Navigation .--..........._-..........-....................-...---..-....- 24 Measurements of flow..................-.....-..-.---......-.-..---...... 25 Susquehanna River at Binghamton, N. Y_-..---...-.-...----.....-..- 25 Ghenango River at Binghamton, N. Y................................ 34 Susquehanna River at Wilkesbarre, Pa......_............-...----_--. 43 Susquehanna River at Danville, Pa..........._..................._... 56 West Branch at Williamsport, Pa .._.................--...--....- _ - - 67 West Branch at Allenwood, Pa.....-........-...-.._.---.---.-..-.-.. 84 Juniata River at Newport, Pa...-----......--....-...-....--..-..---.- -

Otsego County Baseline Water Quality Monitoring1
1 Otsego County baseline water quality monitoring Scott Fickbohm2 INTRODUCTION The following is a preliminary report of water quality data collected between May 2009 and December 2010 at the outflow of Otsego County’s fourteen 11-digit Hydrologic Unit Codes watersheds (HUC’s). While extensive water quality monitoring is currently taking place in the County in specific waterbodies, this effort is meant to be a first step towards being able to characterize baseline water quality across Otsego County by means of direct measurement. Otsego County is 1,007 square miles in area. Estimates of land use are 71% forest, 27% in agriculture and 2% other (urban/developed). From the 11-digit HUC perspective, that area is divided between 14 distinct watersheds. The boundaries of these watersheds extend beyond the County borders and total an area of 1,390 square miles that all drain to the Susquehanna River and, ultimately, to the Chesapeake Bay. Approximately 27 square miles (5%) of Otsego County drains to the Mohawk River Basin through Canajoharie Creek and Cobleskill Creek. These Creeks were not sampled. An exception to the 11-digit HUC approach is the Butternut Creek & Lower Unadilla watersheds. At the 11 HUC level, the Butternut is limited to the area above Morris, NY with the lower portion being considered part of the Lower Unadilla watershed. In order to capture watershed specific data to the greatest extent possible, the Butternut was sampled just north of its confluence with the Unadilla River. The area for each of these watersheds was recalculated based on this sampling point. The names of each watershed sampled, along with their HUC number and area, are provided in the Table 1. -
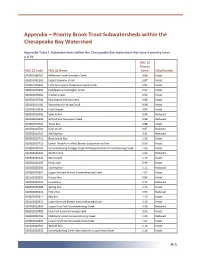
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

A Directory of Primary and Community Resources in the PROBE Area
DOCUMENT RESUME ED 079 201 SO 005 974 AUTHOR Whitehill, Willian E., Jr., Comp.; And Others TITLE A Directory of Primary and Community Resources in the PROBE Area.. INSTITUTION Catskill Area School Study Council,_Oneonta, N.Y.; Otsego County Board of Cooperative Educational Services, Oneonta, N.Y. SPONS AGENCY Office of Education (DHEW), Washington, D.C. Projects to Advance Creativity in Education. - PUB DATE 68 NOTE 210p. EDRS PRICE' MF-$0.65 HC-$9.87 DESCRIPTORS *Community Resources; *Directories; Elementary Education; Resource Guides; Secondary Education; *Social Studies; Teacher Developed Materials IDENTIFIERS ESEA Title III; New York; *Project Probe ABSTRACT Community and area resources -- consisting of persons, places, or objects -- gathered from the regions comprising Chenango,.Delaware, and Otsego Countries, are listed in this directory. Teachers, local historians, and PROBE staff identified resources which could be introduced to K-12 teachers. In addition to a brief introduction, the book contains two major chapters, the first containing lists of primary sources and cultural and educational resources in communities, arranged by county and tLen by business or industry, church, historical sites, libraries, local organizations, museums, public buildings, private collections and schools. Human resources include the local historian, political figures, and other significant persons. The second major chapter, a specialized section, -offers listings of geologidal resources in the three counties; an archeological survey and description -

The Town of Maryland 2019 Comprehensive Plan
The Town of Maryland 2019 Comprehensive Plan Modified January 19, 2019 1 Acknowledgements Harold Palmer, Supervisor, Town of Maryland Danny Lapin, Environmental Planner, Otsego County Conservation Association Shane Digan, Intern, Otsego County Conservation Association Maryland Town Board Harold Palmer, Supervisor, Town of Maryland Christopher Di Donna, Councilman, Town of Maryland Reneé Gaston, Councilwoman, Town of Maryland Scott Gaston, Councilman, Town of Maryland Brian Bookhout, Councilman, Town of Maryland Town of Maryland Department Heads Paul Neske, Zoning Enforcement Officer/Fire Chief Pete Andrews, Former Water Superintendent Tim Walke, Highway Superintendent John Arnold, Town Assessor/Planning Board Chairman Otsego County Board of Representatives Peter Oberacker, Otsego County Representative District 6 Town of Maryland Comprehensive Plan Steering Committee Steve Barton, Chairman Eleanor Stromberg, Member Jason Knapp, Member Barbara Dulkis, Member Gary DuBois, Member Ted Stuka, Member Connie Lull, Member In memory of Scott Roland, member. Schenevus Central School Deb Anderson’s Class 2 Table of Contents Section 1.0: Introduction ............................................................................................................................................................................. 6 Section 1.1: Vision Statement ................................................................................................................................................................ 6 Section 1.2: What is a Comprehensive -

Summer 2019 Newsletter
August 2019 Volume 1, Issue 24 967 County Hwy 33 Cooperstown, NY 13326 Phone (607) 547-8337 ext. 4 Summer Newsletter website: www.otsegosoilandwater.com www.facebook.com/OtsegoSWCD 2019 Leatherstocking Envirothon at Gilbert Lake State Park INSIDE THIS ISSUE From the Manager's Desk................ 2 Cover Crops ...................................... 4 Stream Health .................................. 6 NRCS staff, FSA staff, and SWCD staff gathered for the Oakwilt & Frogbit ............................ 8 dedication of the Robert G. Weaver memorial garden Wild Parsnip & Buttercup ............... 10 NRCS Controlling Water ................. 12 FSA Updates ..................................... 14 Contact Us………………………………….16 ONC Boces students helped plant seedlings at the Forest of the Dozen Dads in Middlefield, NY We will be having a 2020 Tree and Shrub Sale. Cleanup of Schenevus Creek Pre-order forms will be made available in the spring to volunteers “All Hands on Deck Day” ensure you get the species you desire. Conservation—Development—Self-Government From the Desk of SWCD’s Manager 2019 Leatherstocking Envirothon The Leatherstocking Envirothon has been in existence for over 23 years. As fall approaches it’s a stark reminder that spring is not far off. Each spring Otsego County holds the Leatherstocking Envirothon. There is a tremendous effort by OCSWCD staff, numerous volunteers, and donations that make this event possible. We are looking for volunteers and donations to help us make this program a great experience for the students that participate. The day started out with a little drizzle of rain but it turned out to be a beautiful and fun day for all involved. We are striving to continue to grow by adding students and volunteers to this years event. -

Aquatic Invasive Species Present in Otsego County, NY Water Bodies
Aquatic invasive species present in Otsego County, NY water bodies Annie Yoo1, Kaylee Herzog2, and Holly Waterfield CLM3 INTRODUCTION Otsego County, located in central New York, experiences increased populations in the summer months largely related to tourism and outdoor recreation. Abundant water resources in the county, combined with this increase in summer traffic contribute to the risk of invasive species transport between water bodies and major watersheds. Knowledge of the current distribution of invasive species will allow natural resource managers to prioritize transport prevention and population control measures. Exotic invasive species are those not native to an area that outcompete native species. Such exotic, non-native species typically possess traits which, in combination with lack of natural disease and/or predators, allow them to out-compete native species (Santos et al. 2011) and in-so-doing have negative ecological and economic impacts. Some of these impacts include productivity losses in agriculture, forestry, and other segments of the U.S. economy (Pimentel 2005). Aquatic invasive species (AIS) pose major threats to biodiversity in ecosystems. They often overtake important native aquatic plants and animals, cause habitat degradation and loss, and interfere with water-based recreational activities (Zhang and Boyle 2010). Pimentel (2005) suggests it is difficult to estimate the full extent of the environmental damages caused by invasive species and the number of species extinctions they have caused because little is known about each of the ~750,000 species present in the United States. In most cases AIS are initially introduced to watersheds through recreational boating activities or unintentional “hitchhiking ” (Horvath 2008), which can occur through international trade, with invaders stowed in ships, planes, trucks, or packing materials (McNeely 2001). -

NY State Highway Bridge Data: August 31, 2021
NY State Highway Bridge Data: August 31, 2021 Otsego County Year Date BIN Built or of Last Poor Region County Municipality Location Feature Carried Feature Crossed Owner Replaced Inspectio Status n 09 Otsego Brookfield (Town) 3354020 HAMLET OF W EDMESTON WELCH ROAD UNADILLA RIVER 30 - County 1932 07/28/2020 N 09 Otsego Burlington (Town) 1026440 3 MI N WEST BURLINGTON 51 51 94021280 WHARTON CREEK NYSDOT 1994 07/13/2020 N 09 Otsego Burlington (Town) 1026450 2.3 MI SW OF WEST EXETER 51 51 94021302 BINGHAM RD CREEK NYSDOT 1934 05/29/2019 N 09 Otsego Burlington (Town) 1030850 0.5 MI W WEST BURLINGTON 80 80 94041102 WHARTON CREEK NYSDOT 1994 04/21/2020 N 09 Otsego Burlington (Town) 1030860 AT BURLINGTON 80 80 94041145 BUTTERNUT CREEK NYSDOT 2016 11/18/2020 N 09 Otsego Burlington (Town) 3369520 2 MI N BURLINGTON FLATS COUNTY ROAD 19 TRIB. TO WHARTON CREEK 30 - County 1998 08/05/2020 N 09 Otsego Burlington (Town) 3369530 3 MI N BURLINGTON FLATS COUNTY ROAD 19 TRIB WHARTON CREEK 30 - County 1998 08/05/2020 N 09 Otsego Burlington (Town) 3354100 1.2 MI N BURLINGTON FLATS COUNTY ROAD 19 WHARTON CREEK 30 - County 2004 09/09/2020 N 09 Otsego Burlington (Town) 2227350 2.75 NE OF GARRATSVILLE MILLER ROAD BUTTERNUT CREEK 40 - Town 1900 04/26/2021 N 09 Otsego Burlington (Town) 2227370 2.9 MI SE OF WEST EXETER MUNSON ROAD DUNDEE BROOK 40 - Town 1945 03/26/2021 Y 09 Otsego Butternuts (Town) 1026400 2.5 MI NE JCT SH 51 & SH 51 51 94021024 COPES BROOK NYSDOT 1968 07/15/2020 N 09 Otsego Butternuts (Town) 1026420 2 MI NE OF GILBERTSVILLE 51 51 94021069 THORP -

Surface Water Quality in Otsego County, NY, Prior to Potential Natural Gas Exploration
Surface water quality in Otsego County, NY, prior to potential natural gas exploration 1 Sarah Crosier Abstract –Baseline water quality data was established for Otsego County, NY prior to potential natural gas exploration. Hydraulic fracturing may contaminate surface water with chemicals, salts and sediments. Averages for pH, total dissolved solids, and conductivity were calculated for 50 Otsego County streams from data collected using a YSI® multi-parameter probe from August 2010 – April 2012. Limestone bedrock sub-watersheds had significantly higher conductivity, TDS and pH than shale bedrock sub-watersheds. Winter and summer peaks and fall and spring lows occurred in conductivity and TDS. Road salt use, precipitation and evaporation likely caused seasonal variation. Sub-watershed size had no significant effect on parameters. These data will serve as a control for future water quality testing if hydraulic fracturing occurs in Otsego County, NY. INTRODUCTION This study was conducted to identify baseline water quality conditions at base flow for streams in Otsego County. Water quality varies between streams due to different physical, chemical, and microbiological characteristics (Rajeshwari and Saraswathi 2009). These factors are dependent upon topography, geology, vegetative cover (Dosskey et al. 2010), land use in a watershed (Ou and Wang 2011) and watershed size (Landers et al. 2007). Stream chemistry varies naturally throughout the seasons due to changes in precipitation, evaporation, nutrient input, and biotic activity within the streams. Anthropogenic pollutants such as road salt (Jackson and Jobbágy 2005, Kaushal et al. 2005), urban storm water runoff and agricultural pesticides, nutrients, and sediments (Madden et al. 2007) also affect stream quality variably. -

Baseline Surface Water Monitoring in Otsego County
Acknowledgements I would like to thank the following people and departments for their assistance in making this project possible. This research was partially funded by the Otsego County Conservation Association through Scott Fickbohm at the Otsego County Soil and Water Conservation District. Other funding came from the Departments of Chemistry, and Geology and Environmental Sciences at Hartwick College. I thank Dr. John Dudek, who assisted me with instrumentation throughout my analytical work and Dr. Zsuzsanna Balogh-Brunstad, who served as a mentor and thesis advisor for this project. ii Baseline Water Quality Monitoring in the Watersheds of Otsego County, NY by Nicole M. Daniels, B.A. Hartwick College May 2013 Advisor: Dr. Zsuzsanna Balogh-Brunstad Abstract The Marcellus Shale has become of particular interest to those interested in natural gas production using the now economically feasible hydraulic fracturing (fracking) technique. The Otsego County Soil and Water Conservation District has begun monitoring local watersheds so that a baseline for various parameters (pH, turbidity, total dissolved solid, temperature, and electrical conductivity) can be set. This way, if the Marcellus Shale in New York State is selected for natural gas extraction using hydraulic fracturing methods, the water quality can be compared to pre-gas extraction levels to insure the integrity of the water quality and ecosystem. The goal of this study was to determine the current concentrations of strontium in surface water in Otsego County, NY as it is an indicator of brine water input to freshwater ecosystems. Brines are associated with flowback and production waters of natural gas extraction after fracking operations, and thus, can indicate a mishandling of such fluids on the surface. -
SUNY, College at Oneonta the Geomorpbology of East-Central New
A-5 GLACIAL MORPHOLOGY OF UPPER SUSQUEllAJlNA DRAINAGE P . Jay Fleisher SUNY , College at Oneonta INTRODUCTION The geomorpbology of east-central New York State reveals the cumulati ve erosional effects of multiple glacial events. but the deposits of only the last ice sheet are known . Evidence for pre-Wisconsin glaciation has long been recognized along the glacial limit in Pennsylvania and from isolated and widely spaced localities in New York. However, the glacieJ. chronology of the eastern Appalachian Plateau is confined to subdivis ions of the Wisconsin Glaciation as displayed in the landforms and stratigraphy. Those factors that influenced glacier pulses , floW' regime. and ice- marginal activity were widely variable across New York State, resulting in problems of correlation and chronology . In spite o~ this, a compre hensive picture has been developed through the combined efforts of many contributors who have concentrated on specific areas~ drainages, and problems. Within tbe area of the upper Susquebanna drainage are the depos its of mid-Wisconsin to late Wisconsin deglaciation . A characteristic • assemblage of depositional landforms const i tutes the valley f l oor morphology and r epresents a par ticular envir onment of deglacial processes. The valley wa.1.ls and divides are , for the most part, examples of the combined effects of erosional and depositional conditions of a different glacial environment. The emphasis of thi s report will be to consider the glacier environments of deposition as represented by the landforms and their stratigraphy. The area under consideration lies within Otsego County. along the upper reaches of the Susquehanna River from Wells Bridge to Otsego Lake.