King Island Biodiversity Management Plan 2012–2022
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Relationships of Discyphus Scopulariae
Phytotaxa 173 (2): 127–139 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.173.2.3 Phylogenetic relationships of Discyphus scopulariae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences: evidence supporting recognition of a new subtribe, Discyphinae GERARDO A. SALAZAR1, CÁSSIO VAN DEN BERG2 & ALEX POPOVKIN3 1Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70-367, 04510 México, Distrito Federal, México; E-mail: [email protected] 2Universidade Estadual de Feira de Santana, Departamento de Ciências Biológicas, Av. Transnordestina s.n., 44036-900, Feira de Santana, Bahia, Brazil 3Fazenda Rio do Negro, Entre Rios, Bahia, Brazil Abstract The monospecific genus Discyphus, previously considered a member of Spiranthinae (Orchidoideae: Cranichideae), displays both vegetative and floral morphological peculiarities that are out of place in that subtribe. These include a single, sessile, cordate leaf that clasps the base of the inflorescence and lies flat on the substrate, petals that are long-decurrent on the column, labellum margins free from sides of the column and a column provided with two separate, cup-shaped stigmatic areas. Because of its morphological uniqueness, the phylogenetic relationships of Discyphus have been considered obscure. In this study, we analyse nucleotide sequences of plastid and nuclear DNA under maximum parsimony -

Australia Lacks Stem Succulents but Is It Depauperate in Plants With
Available online at www.sciencedirect.com ScienceDirect Australia lacks stem succulents but is it depauperate in plants with crassulacean acid metabolism (CAM)? 1,2 3 3 Joseph AM Holtum , Lillian P Hancock , Erika J Edwards , 4 5 6 Michael D Crisp , Darren M Crayn , Rowan Sage and 2 Klaus Winter In the flora of Australia, the driest vegetated continent, [1,2,3]. Crassulacean acid metabolism (CAM), a water- crassulacean acid metabolism (CAM), the most water-use use efficient form of photosynthesis typically associated efficient form of photosynthesis, is documented in only 0.6% of with leaf and stem succulence, also appears poorly repre- native species. Most are epiphytes and only seven terrestrial. sented in Australia. If 6% of vascular plants worldwide However, much of Australia is unsurveyed, and carbon isotope exhibit CAM [4], Australia should host 1300 CAM signature, commonly used to assess photosynthetic pathway species [5]. At present CAM has been documented in diversity, does not distinguish between plants with low-levels of only 120 named species (Table 1). Most are epiphytes, a CAM and C3 plants. We provide the first census of CAM for the mere seven are terrestrial. Australian flora and suggest that the real frequency of CAM in the flora is double that currently known, with the number of Ellenberg [2] suggested that rainfall in arid Australia is too terrestrial CAM species probably 10-fold greater. Still unpredictable to support the massive water-storing suc- unresolved is the question why the large stem-succulent life — culent life-form found amongst cacti, agaves and form is absent from the native Australian flora even though euphorbs. -

Breeding System Diversification and Evolution in American Poa Supersect. Homalopoa (Poaceae: Poeae: Poinae)
Annals of Botany Page 1 of 23 doi:10.1093/aob/mcw108, available online at www.aob.oxfordjournals.org Breeding system diversification and evolution in American Poa supersect. Homalopoa (Poaceae: Poeae: Poinae) Liliana M. Giussani1,*, Lynn J. Gillespie2, M. Amalia Scataglini1,Marıa A. Negritto3, Ana M. Anton4 and Robert J. Soreng5 1Instituto de Botanica Darwinion, San Isidro, Buenos Aires, Argentina, 2Research and Collections Division, Canadian Museum of Nature, Ottawa, Ontario, Canada, 3Universidad de Magdalena, Santa Marta, Colombia, 4Instituto Multidisciplinario de Biologıa Vegetal (IMBIV), CONICET-UNC, Cordoba, Argentina and 5Department of Botany, Smithsonian Institution, Washington, DC, USA *For correspondence. E-mail [email protected] Received: 11 December 2015 Returned for revision: 18 February 2016 Accepted: 18 March 2016 Downloaded from Background and Aims Poa subgenus Poa supersect. Homalopoa has diversified extensively in the Americas. Over half of the species in the supersection are diclinous; most of these are from the New World, while a few are from South-East Asia. Diclinism in Homalopoa can be divided into three main types: gynomonoecism, gynodioe- cism and dioecism. Here the sampling of species of New World Homalopoa is expanded to date its origin and diver- sification in North and South America and examine the evolution and origin of the breeding system diversity. Methods A total of 124 specimens were included in the matrix, of which 89 are species of Poa supersect. http://aob.oxfordjournals.org/ Homalopoa sections Acutifoliae, Anthochloa, Brizoides, Dasypoa, Dioicopoa, Dissanthelium, Homalopoa sensu lato (s.l.), Madropoa and Tovarochloa, and the informal Punapoa group. Bayesian and parsimony analyses were conducted on the data sets based on four markers: the nuclear ribosomal internal tanscribed spacer (ITS) and exter- nal transcribed spacer (ETS), and plastid trnT-L and trnL-F. -

National Parks and Wildlife Act 1972.PDF
Version: 1.7.2015 South Australia National Parks and Wildlife Act 1972 An Act to provide for the establishment and management of reserves for public benefit and enjoyment; to provide for the conservation of wildlife in a natural environment; and for other purposes. Contents Part 1—Preliminary 1 Short title 5 Interpretation Part 2—Administration Division 1—General administrative powers 6 Constitution of Minister as a corporation sole 9 Power of acquisition 10 Research and investigations 11 Wildlife Conservation Fund 12 Delegation 13 Information to be included in annual report 14 Minister not to administer this Act Division 2—The Parks and Wilderness Council 15 Establishment and membership of Council 16 Terms and conditions of membership 17 Remuneration 18 Vacancies or defects in appointment of members 19 Direction and control of Minister 19A Proceedings of Council 19B Conflict of interest under Public Sector (Honesty and Accountability) Act 19C Functions of Council 19D Annual report Division 3—Appointment and powers of wardens 20 Appointment of wardens 21 Assistance to warden 22 Powers of wardens 23 Forfeiture 24 Hindering of wardens etc 24A Offences by wardens etc 25 Power of arrest 26 False representation [3.7.2015] This version is not published under the Legislation Revision and Publication Act 2002 1 National Parks and Wildlife Act 1972—1.7.2015 Contents Part 3—Reserves and sanctuaries Division 1—National parks 27 Constitution of national parks by statute 28 Constitution of national parks by proclamation 28A Certain co-managed national -
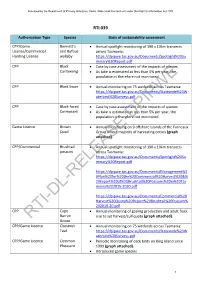
Rti-Dl-Release-Dpipwe
Assessed by the Department of Primary Industries, Parks, Water and Environment under the Right to Information Act 2009 RTI 039 Authorisation Type Species Basis of sustainability assessment CPP/Game Bennett’s • Annual spotlight monitoring of 190 x 10km transects Licence/Commercial and Rufous across Tasmania: Hunting Licence wallaby https://dpipwe.tas.gov.au/Documents/Spotlight%20Su mmary%20Report.pdf CPP Black • Case by case assessment of the impacts of species Currawong • As take is estimated at less than 5% per year, the population is therefore not monitored. CPP Black Swan • Annual monitoring on 75 wetlands across Tasmania: https://dpipwe.tas.gov.au/Documents/Statewide%20W aterbird%20Surveys.pdf CPP Black-faced • Case by case assessment of the impacts of species Cormorant • As take is estimated at less than 5% per year, the population is therefore not monitored. Game Licence Brown • Annual monitoring on 9 offshore islands of the Furneaux Quail Group where majority of harvesting occurs (graph attached). CPP/Commercial Brushtail • Annual spotlight monitoring of 190 x 10km transects possum across Tasmania: https://dpipwe.tas.gov.au/Documents/Spotlight%20Su mmary%20Report.pdf https://dpipwe.tas.gov.au/Documents/Management%2 0Plan%20for%20the%20Commercial%20Harvest%20&% 20Export%20of%20Brushtail%20Possums%20in%20Tas mania%202015-2020.pdf https://dpipwe.tas.gov.au/Documents/Commercial%20 Harvest%20Quota%20Report%20Brushtail%20Possum% 202019-20.pdf CPP Cape • Annual monitoring of gosling production and adult flock Barren size to set harvest/cull quota (graph attached). RTI-DL-RELEASE-DPIPWEGoose CPP/Game Licence Chestnut • Annual monitoring on 75 wetlands across Tasmania: Teal https://dpipwe.tas.gov.au/Documents/Statewide%20W aterbird%20Surveys.pdf CPP/Game Licence Common • Periodic monitoring of cock birds on King Island since Pheasant 1999 (graph attached). -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -

Special Issue3.7 MB
Volume Eleven Conservation Science 2016 Western Australia Review and synthesis of knowledge of insular ecology, with emphasis on the islands of Western Australia IAN ABBOTT and ALLAN WILLS i TABLE OF CONTENTS Page ABSTRACT 1 INTRODUCTION 2 METHODS 17 Data sources 17 Personal knowledge 17 Assumptions 17 Nomenclatural conventions 17 PRELIMINARY 18 Concepts and definitions 18 Island nomenclature 18 Scope 20 INSULAR FEATURES AND THE ISLAND SYNDROME 20 Physical description 20 Biological description 23 Reduced species richness 23 Occurrence of endemic species or subspecies 23 Occurrence of unique ecosystems 27 Species characteristic of WA islands 27 Hyperabundance 30 Habitat changes 31 Behavioural changes 32 Morphological changes 33 Changes in niches 35 Genetic changes 35 CONCEPTUAL FRAMEWORK 36 Degree of exposure to wave action and salt spray 36 Normal exposure 36 Extreme exposure and tidal surge 40 Substrate 41 Topographic variation 42 Maximum elevation 43 Climate 44 Number and extent of vegetation and other types of habitat present 45 Degree of isolation from the nearest source area 49 History: Time since separation (or formation) 52 Planar area 54 Presence of breeding seals, seabirds, and turtles 59 Presence of Indigenous people 60 Activities of Europeans 63 Sampling completeness and comparability 81 Ecological interactions 83 Coups de foudres 94 LINKAGES BETWEEN THE 15 FACTORS 94 ii THE TRANSITION FROM MAINLAND TO ISLAND: KNOWNS; KNOWN UNKNOWNS; AND UNKNOWN UNKNOWNS 96 SPECIES TURNOVER 99 Landbird species 100 Seabird species 108 Waterbird -

Great Australian Bight BP Oil Drilling Project
Submission to Senate Inquiry: Great Australian Bight BP Oil Drilling Project: Potential Impacts on Matters of National Environmental Significance within Modelled Oil Spill Impact Areas (Summer and Winter 2A Model Scenarios) Prepared by Dr David Ellis (BSc Hons PhD; Ecologist, Environmental Consultant and Founder at Stepping Stones Ecological Services) March 27, 2016 Table of Contents Table of Contents ..................................................................................................... 2 Executive Summary ................................................................................................ 4 Summer Oil Spill Scenario Key Findings ................................................................. 5 Winter Oil Spill Scenario Key Findings ................................................................... 7 Threatened Species Conservation Status Summary ........................................... 8 International Migratory Bird Agreements ............................................................. 8 Introduction ............................................................................................................ 11 Methods .................................................................................................................... 12 Protected Matters Search Tool Database Search and Criteria for Oil-Spill Model Selection ............................................................................................................. 12 Criteria for Inclusion/Exclusion of Threatened, Migratory and Marine -

Native Orchid Society of South Australia Inc
Native Orchid Society of South Australia Inc. Journal Diuris calcicola One of new orchid species named in 2015 Photo: R. Bates June 2016 Volume 40 No. 5 Native Orchid Society of South Australia June 2016 Vol. 40 No. 5 The Native Orchid Society of South Australia promotes the conservation of orchids through preservation of natural habitat and cultivation. Except with the documented official representation of the management committee, no person may represent the Society on any matter. All native President orchids are protected in the wild; their collection without written Vacant Government permit is illegal. Vice President Robert Lawrence Contents Email: [email protected] Title Author Page Secretary Bulletin Board 54 Rosalie Lawrence Vice President’s Report Robert Lawrence 55 Email:[email protected] May Field Trip – From a newbie Vicki Morris 56 Treasurer NOSSA Seed Kits 2016 Les Nesbitt 57 Christine Robertson The Orchid & Mycorrhiza Fungus… Rob Soergel 57 Email: [email protected] Editor Growing Exercise Recall Les Nesbitt 58 Lorraine Badger Diuris Project Report Les Nesbitt 58 Assistant Editor - Rob Soergel May Meeting Review Rob Soergel 58 Email: [email protected] Pterostylis - Reprint 59 Committee Letters to the editor 60 Michael Clark May Orchid Pictures Competition Rosalie Lawrence 62 Bob Bates April Benched Orchids Results Les Nesbitt 63 Kris Kopicki April Benched Orchids Photos Judy & Greg Sara 64 Other Positions Membership Liaison Officer Life Members Robert Lawrence Mr R Hargreaves† Mr G Carne Mrs T Bridle Ph: 8294 8014 Email:[email protected] Mr H Goldsack† Mr R Bates Botanical Advisor Mr R Robjohns† Mr R Shooter Bob Bates Mr J Simmons† Mr W Dear Conservation Officer Mr D Wells† Mrs C Houston Thelma Bridle Ph: 8384 4174 Mr L Nesbitt Mr D Hirst Field Trips Coordinator Michael Clark Patron: Mr L. -

Poa Billardierei
Poa billardierei COMMON NAME Sand tussock, hinarepe SYNONYMS Festuca littoralis Labill.; Schedonorus littoralis (Labill.) P.Beauv.; Triodia billardierei Spreng.; Poa billardierei (Spreng.)St.-Yves; Schedonorus billardiereanus Nees; Arundo triodioides Trin.; Schedonorus littoralis var. alpha minor Hook.f.; Austrofestuca littoralis (Labill.) E.B.Alexev. FAMILY Poaceae AUTHORITY Poa billardierei (Spreng.)St.-Yves FLORA CATEGORY Vascular – Native ENDEMIC TAXON No Austrofestuca littoralis. Photographer: Kevin Matthews ENDEMIC GENUS No ENDEMIC FAMILY No STRUCTURAL CLASS Grasses NVS CODE POABIL CHROMOSOME NUMBER 2n = 28 CURRENT CONSERVATION STATUS 2012 | At Risk – Declining | Qualifiers: SO PREVIOUS CONSERVATION STATUSES 2009 | At Risk – Declining | Qualifiers: SO 2004 | Gradual Decline DISTRIBUTION Austrofestuca littoralis. Photographer: Geoff North Island, South Island, Chatham Island (apparently absent from Walls Chatham Island now despite being formerly abundant). Also found in temperate Australia. HABITAT Coastal dunes; sandy and rocky places near the shore, especially foredunes and dune hollows. FEATURES Yellow-green tussocks up to about 70 cm tall. Leaves fine, rolled, somewhat drooping (coarser than silver tussock), initially green, often fading at tips to silver, and drying to golden-straw colour. Seed heads no longer than leaves; seeds relatively large, barley-like, leaving a characteristic zig-zag look to the remaining head when fallen. Flowers in early summer and the seed are produced in late summer. It could be confused with Poa chathamica which has blue- green or grass-green flat leaves and an open seed head which overtops the foliage. It could also be confused with marram grass which has similar foliage but large cat’stail-like seed heads which overtop the foliage. SIMILAR TAXA Ammophila arenaria (marram grass) is often confused with sand tussock because they grow in the same habitat. -

Introduced Weed Species
coastline Garden Plants that are Known to Become Serious Coastal Weeds SOUTH AUSTRALIAN COAST PROTECTION BOARD No 34 September 2003 GARDEN PLANTS THAT HAVE BECOME Vegetation communities that originally had a diverse SERIOUS COASTAL WEEDS structure are transformed to a simplified state where Sadly, our beautiful coastal environment is under threat one or several weeds dominate. Weeds aggressively from plants that are escaping from gardens and compete with native species for resources such as becoming serious coastal weeds. Garden escapees sunlight, nutrients, space, water, and pollinators. The account for some of the most damaging environmental regeneration of native plants is inhibited once weeds are weeds in Australia. Weeds are a major environmental established, causing biodiversity to be reduced. problem facing our coastline, threatening biodiversity and the preservation of native flora and fauna. This Furthermore, native animals and insects are significantly edition of Coastline addresses a selection of common affected by the loss of indigenous plants which they rely garden plants that are having significant impacts on our on for food, breeding and shelter. They are also affected coastal bushland. by exotic animals that prosper in response to altered conditions. WHAT ARE WEEDS? Weeds are plants that grow where they are not wanted. Weeds require costly management programs and divert In bushland they out compete native plants that are then resources from other coastal issues. They can modify excluded from their habitat. Weeds are not always from the soil and significantly alter dune landscapes. overseas but also include native plants from other regions in Australia. HOW ARE WEEDS INTRODUCED AND SPREAD? WEEDS INVADE OUR COASTLINE… Weeds are introduced into the natural environment in a Unfortunately, introduced species form a significant variety of ways. -

Evolutionary Shifts in Fruit Dispersal Syndromes in Apiaceae Tribe Scandiceae
Plant Systematics and Evolution (2019) 305:401–414 https://doi.org/10.1007/s00606-019-01579-1 ORIGINAL ARTICLE Evolutionary shifts in fruit dispersal syndromes in Apiaceae tribe Scandiceae Aneta Wojewódzka1,2 · Jakub Baczyński1 · Łukasz Banasiak1 · Stephen R. Downie3 · Agnieszka Czarnocka‑Cieciura1 · Michał Gierek1 · Kamil Frankiewicz1 · Krzysztof Spalik1 Received: 17 November 2018 / Accepted: 2 April 2019 / Published online: 2 May 2019 © The Author(s) 2019 Abstract Apiaceae tribe Scandiceae includes species with diverse fruits that depending upon their morphology are dispersed by gravity, carried away by wind, or transported attached to animal fur or feathers. This diversity is particularly evident in Scandiceae subtribe Daucinae, a group encompassing species with wings or spines developing on fruit secondary ribs. In this paper, we explore fruit evolution in 86 representatives of Scandiceae and outgroups to assess adaptive shifts related to the evolutionary switch between anemochory and epizoochory and to identify possible dispersal syndromes, i.e., patterns of covariation of morphological and life-history traits that are associated with a particular vector. We also assess the phylogenetic signal in fruit traits. Principal component analysis of 16 quantitative fruit characters and of plant height did not clearly separate spe- cies having diferent dispersal strategies as estimated based on fruit appendages. Only presumed anemochory was weakly associated with plant height and the fattening of mericarps with their accompanying anatomical changes. We conclude that in Scandiceae, there are no distinct dispersal syndromes, but a continuum of fruit morphologies relying on diferent dispersal vectors. Phylogenetic mapping of ten discrete fruit characters on trees inferred by nrDNA ITS and cpDNA sequence data revealed that all are homoplastic and of limited use for the delimitation of genera.