The Influence of Pupil Responses on Subjective Brightness Perception
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Intracameral Pupil-Dilating Technique
AN INTRACAMERAL PUPIL-DILATING TECHNIQUE No devices are needed with this reversible method. BY MOHAMMAD IDREES, FRCS(EDIN) CATARACT SURGERY CATARACT In cataract surgery, a sufficiently dilated associated with glare. Moreover, chances of damage to pupil is necessary to allow the surgeon to the capsular margin are increased due to poor visibility. safely remove the cataract and replace it Emulsification risks damage to the posterior lens capsule, with an appropriate IOL. When the pupil which opens the door to other complications later. is not sufficiently dilated, it obstructs the It is difficult to get a sufficient red fundus reflex with a surgeon’s view and makes each step more poorly dilating pupil. Implanting the IOL poses its own set difficult, increasing the risk of complications of potential complications, such as decentration and subse- during surgery and unfavorable outcomes quent malpositioning of the IOL. postoperatively. Operating through a small pupil can result in iris bleeding, These increased risks can be avoided if the pupil is dilated iris prolapse into the wound, or incomplete evacuation of well before starting surgery. This article describes a reversible cortical material. Additionally, iris chafing can contribute to method that I call the Idrees intracameral pupil-dilating technique increased postoperative inflammation and iris defects that and presents the rationale for its use. can create cosmesis concerns. SURGICAL RISKS WITH SMALL PUPILS PUPIL DILATION: MECHANISM AND TECHNIQUE Among the most prominent challenges with a narrow In order to achieve a satisfactorily dilated pupil in every case, pupil, performing capsulorrhexis or anterior capsulotomy of you must have a number of approaches at your disposal; some sufficient diameter is not possible. -

Anatomy & Physiology of The
Anatomy & Physiology of The Eye 2017-2018 Done By: 433 Team Abdullah M. Khattab Important Doctor’s Notes Extra Abdullah AlOmair Resources: Team 433, Doctors Notes, Vaughan & Asbury’s General ophthalmology. Editing File Embryology of The Eye ............................................................................................. 2 ● Defects: ........................................................................................................................... 2 Development of The Eye After Birth .......................................................................... 3 ● Refractive power depends on two factors: ...................................................................... 3 The Orbit ................................................................................................................... 4 ● Seven bones contribute the bony orbit and surrounded by nasal sinuses. .................... 4 ● The orbital wall, pear-like shaped, formed by: ................................................................ 4 ● Structures Passing Through the Optic Openings: ........................................................... 4 Extraocular Muscles .................................................................................................. 1 ● Anatomy .......................................................................................................................... 1 ● Notes: .............................................................................................................................. 1 ● Field of action: -

Download Our Coloboma Factsheet In
Coloboma Factsheet Contents 3 What is Coloboma? 3 How do we see with our eyes? 3 Which parts of the eye can coloboma affect? 3 Iris 4 Lens zonules 4 Retina and choroid (chorioretinal) 4 Optic disc 4 Eyelids 4 What causes coloboma to form inside the eye? 5 Does coloboma affect vision? 5 Iris coloboma 5 Lens coloboma 5 Chorioretinal coloboma 6 How is coloboma diagnosed? 7 What is the treatment for coloboma? 7 Can coloboma lead to other eye health problems? 7 Glaucoma 8 Retinal detachment 8 Choroidal neovascularisation (new blood vessels) 8 Cataract 9 What other health problems can affect some children with coloboma? 9 Coping with sight problems relating to coloboma 10 Further help and support 10 Sources of support 11 Other useful organisations 12 We value your feedback 2 What is Coloboma? Coloboma means that part of one or more structures Choroid inside an unborn baby’s eye does not fully develop Iris during pregnancy. This underdeveloped tissue is Optic nerve normally in the lower part of the eye and it can be Optic Cornea small or large in size. A coloboma occurs in about 1 in disc 10,000 births and by the eighth week of pregnancy. Macula Lens Coloboma can affect one eye (unilateral) or both eyes zonules Pupil (bilateral) and it can affect different parts of the eye. As coloboma forms during the initial development of Choroid the eye, it is present from birth and into adulthood. Lens Retina How do we see with our eyes? Iris Light enters our eyes by passing through our cornea, our pupil, (the hole in the middle of the iris), and Diagram of cross section of eye (labels cornea, lens, iris, our lens so that it is sharply focused onto the retina vitreous humour, macula, retina, choroid, optic nerve) lining the back of our eye. -

The Oval Pupil
Eye (1991) 5, 466-469 The Oval Pupil H. S. DUA. N. J. WATSON. J. Y. FORRESTER Ahf'/'{/ecn Summary The dynamics of pupillary dilation induced by Phenylephrine 10% and Cyclopento late 1 % have been examined by flash photography. A correlation between anterior chamber depth and the pupil shape on dilation with Phenylephrine Hydrochloride J00/r, is described. It is postulated that these pupillary dilation dynamics support a s)'mpathetic abnormality as a trigger for acute primary angle closure glaucoma. The oval pupil is a common clinical sign in clinics after ocular and general examination acute prImary angle closure glaucoma had excluded conditions kno wn to affect (APACG ).1 APACG has been shown to occur ocular sympathetic function. Exclusion cri more commonly in patients with shallO\v teria included diabetes mellitus. glaucoma or anterior chambers.: The acute attack is pre ocular hypertension. previous ocular surgery cipitated by autonomic dysfunction and a (including extraocular muscle surgery). pre modification of physiological pupil block,: vious intraocular inflammation and topical During an attack of APACG the pupil is mid drug administration. The colour of the iris was dilated and vertically elongated. a preferen noted and the anterior chamber depth was tial dilation in the vertical meridian producing measured on a Haag Streit slit lamp using the the oval shape,' Oval or 'tadpole shaped' technique described by Smith,' A tine beam is pupils have also been reported in patients directed horizontally at the eye from 60°, Its with Horner's syndrome.-l As both these con length is adjusted until the corneal and iridal ditions with oval pupils have an underlying reflexes meet. -

Anatomy & Physiology Vocabulary List
Anatomy & Physiology Vocabulary List http://www.preventblindness.org/vlc/how_we_see.htm Anterior Chamber: space in front portion of the eye between the cornea and the iris and lens, which is filled with aqueous humor Aqueous Humor: a clear, watery fluid that fills the front part of the eye between the cornea, lens and iris. Binocular Vision: coordinated use of the two eyes to see a single fused 3D image Choroid: the middle layer of the eyeball which contains veins and arteries that furnishes nourishment to the eye, especially the retina. Conjunctiva: a mucous membrane that lines the eyelids and covers the front part of the eyeball. Cornea: the transparent outer portion of the eyeball that transmits light to the retina. Ciliary Body: a ring of tissue between the iris and the choroid consisting of muscles and blood vessels that changes the shape of the lens and manufactures aqueous humor Fovea: A tiny spot located in the macula that is the area of clearest vision on the retina. Iris: the colored, circular part of the eye in front of the lens. It controls the size of the pupil. Lens: the transparent disc in the middle of the eye behind the pupil that brings rays of light into focus on the retina. Macula: is a small area of the retina located near the optic nerve at the back of the eye. It is responsible for our central, most acute vision. Optic Disk: head of optic nerve, the meeting of all retinal nerve fibers in the retina Optic Nerve: the importantt nerve tha carries messages from the retina to the brain. -

THE PUPIL Alpha Omega Pupil the PUPIL Alpha Omega Pupil
THE PUPIL Observation and Grading Alpha Omega Pupil JohnJohn J. J. Pulaski,Pulaski, O.D.,O.D., FCSOFCSO CollegeCollege ofof SyntonicSyntonic OptometryOptometry 101101 CourseCourse May 2020 Pulaski AO Pupil The Pupil And Syntonics How do you know if a person needs Syntonic Treatment? Pulaski AO Pupil The Pupil And Syntonics Three keys elements in Syntonic Clinical evaluationevaluation andand treatmenttreatment application.application. 1. The Pupil - AO 2.2. The The FieldField -- Kinetic Kinetic 3.3. The The PatientPatient HistoryHistory Pulaski AO Pupil Pulaski AO Pupil The Pupil One of the most sensitive measures of ANS activity. •• ANS/Brainstem ANS/Brainstem functionfunction •• “Eyes “Eyes areare thethe windowwindow toto thethe Soul”Soul” TheThe PupilsPupils areare thethe window.window. •• Portal Portal ofof EnergyEnergy ReceptionReception andand Projection.Projection. PortalPortal throughthrough whichwhich we interact with our world •• Non-verbal Non-verbal CommunicationCommunication andand strongstrong emotionalemotional indicatorindicator .. •• Reception Reception ofof “nutrition”“nutrition” - - LIGHT LIGHT Pulaski AO Pupil Pulaski AO Pupil The Pupil Neurological Pathways ParasympatheticParasympathetic -- Constriction Constriction •The•The PupillaryPupillary LightLight ReflexReflex (PLR)(PLR) •Influence•Influence onon IrisIris SphincterSphincter •Light-Inhibited•Light-Inhibited SympatheticSympathetic PathPath •Trigeminal•Trigeminal NerveNerve –– sensory sensory stimulationstimulation toto eye/iriseye/iris SympatheticSympathetic –– -
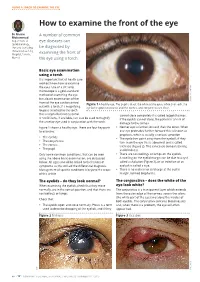
How to Examine the Front of the Eye
USING A TORCH TO EXAMINE THE EYE How to examine the front of the eye Dr Nasiru A number of common Muhammad Department of eye diseases can Ophthalmology, Usmanu Danfodiyo be diagnosed by University Teaching Hospital, Sokoto, examining the front of Nigeria. the eye using a torch. Basic eye examination using a torch It is important that all health care workers know how to examine the eyes. Use of a slit lamp microscope is a gold-standard method of examining the eye but a basic examination of the front of the eye can be carried SANDIP DAS SANYAM Figure 1 A healthy eye. The pupil is black, the white of the eye is white (not red), the out with a torch; if a magnifying eyelashes point outwards and the cornea and conjunctiva are clear. loupe is attached to the torch this is helpful but not essential. cannot close completely it is called lagophthalmos. A +20 DS lens, if available, can also be used to magnify If the eyelids cannot close, the patient is at risk of the anterior eye used in conjunction with the torch. damage to the cornea. Figure 1 shows a healthy eye. There are four key parts • Neither eye is further forward than the other. When to examine: one eye protrudes further forward this is known as proptosis, which is usually a serious condition. • The eyelids • The eyelashes point away from the eyeball; if they • The conjunctiva turn in on the eye this is abnormal and is called • The cornea trichiasis (Figure 2). This can cause corneal scarring • The pupil and blindness. -

Pupillary Response to Representations of Light in Paintings
Journal of Vision (2020) 20(10):14, 1–18 1 Pupillary response to representations of light in paintings Department of Neurofarba, University of Florence, Serena Castellotti Florence, Italy Department of Neurofarba, University of Florence, Martina Conti Florence, Italy Federal University of ABC, Sao Bernardo do Campo, Brazil Claudia Feitosa-Santana Neuroscience for Human Development, Sao Paulo, Brazil Department of Neurofarba, University of Florence, Maria Michela Del Viva Florence, Italy It is known that, although the level of light is the primary determinant of pupil size, cognitive factors can Introduction also affect pupil diameter. It has been demonstrated that photographs of the sun produce pupil constriction The pupil is the central opening of the iris that independently of their luminance and other low-level regulates the intensity of light entering the eye to adjust features, suggesting that high-level visual processing retinal illumination and optimize vision (Loewenfeld, may also modulate pupil response. 1993). Light increments produce pupillary constriction Here, we measure pupil response to artistic paintings (miosis), whereas light decrements produce pupillary of the sun, moon, or containing a uniform lighting, that, dilation (mydriasis). This is known as pupillary light being mediated by the artist’s interpretation of reality reflex (PLR), which is controlled by the autonomic and his technical rendering, require an even higher level nervous system (Gamlin & Clarke, 1995; Loewenfeld, of interpretation compared with photographs. We also 1993). Currently, a consistent body of evidence study how chromatic content and spatial layout affect demonstrates that the PLR is not merely a basic the results by presenting grey-scale and inverted low-level mechanism, showing that, even if the versions of each painting. -

Iris Surface Deformation and Normalization
Iris Surface Deformation and Normalization Somying Thainimit Luís A. Alexandre Vasco M. N. de Almeida Electrical Engineering Department Department of Informatics Department of Physics Faculty of Engineering University of Beira Interior University of Beira Interior, Covilhã Kasetsart University IT-Instituto de Telecomunicações Centre of Physics Bangkok, Thailand Covilhã, Portugal University of Minho, Braga [email protected] [email protected] Portugal [email protected] Abstract—Human iris recognition systems are an attractive form of non-intrusive bio-identification with many potential II. HUMAN IRIS AND PUPIL applications. However, the accuracy of these systems is still limited due to challenges presented by iris surface deformation. A. The Iris This paper provides an introduction to the physiology of the iris, The human iris is a visible color ring bounded by the pupil describes the problem of iris deformation, and presents a review (the dark opening) and white sclera, as depicted in Fig. 1. The of past and current software approaches to iris recognition. The iris is suspended in aqueous humor, behind the cornea but in focus is on methods related to the physiology of the iris. front of the lens as shown in Fig. 2. The iris divides the space between the lens and the cornea into the anterior chamber Keywords—iris surface deformation; iris normalization; iris recognition; (between the cornea and the iris) and posterior chambers (between the iris and the lens). The periphery of the iris is attached to its root at the iridocorneal angle of the anterior I. INTRODUCTION chamber where it merges with the tissue inside the eye: the Deformations of the iris are primary changes that affect ciliary body and trabecular meshwork. -

Retinal Fixation Point Location in the Foveal Avascular Zone
Investigative Ophthalmology & Visual Science, Vol. 31, No. 10, October 1990 Copyright © Association for Research in Vision and Ophthalmology Retinal Fixation Point Location in the Foveal Avascular Zone Bernard 5. Zeffren,* Raymond A. Applegare,* Arthur Bradley,f and W. A. J. van Heuven" The site of normal fixation is often assumed to be centered in the foveal avascular zone (FAZ). This assumed anatomic relationship is used during photocoagulation therapy as an objective guide to avoid damaging critical retinal structures on or near fixation. With laser therapy being directed closer and closer to the center of the FAZ, the accuracy with which the center of the FAZ locates the retinal point of fixation becomes an important therapeutic issue. Using an optimized technique for visualizing the retinal vasculature entoptically, the authors determined the location of the retinal point of fixation with respect to the foveal area vasculature in 26 eyes of 14 healthy subjects. In 23 eyes (12 subjects), a traditional FAZ was observed, the other three eyes (two subjects) had capillaries near or crossing the center of fixation. Of the 23 eyes with a traditional FAZ, 20 had centers of fixation located eccentric to the center but in the FAZ, (average deviation from the center of the FAZ, 66.5 ± 49.5 /xm) with the direction of deviation from the FAZ center appearing random. Consequently, when following protocols that advocate photocoagulation treatment with spot centers closer to the FAZ center than 300 nm, the center of the FAZ is a poor locator of a subject's retinal point of fixation. When using the FAZ as a reference, the resulting uncertainty in the location of the subject's retinal point of fixation increases the probability of significant damage to the actual point of fixation by up to 20%. -
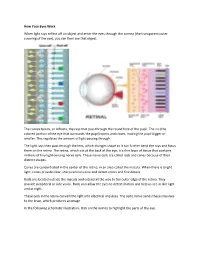
How Your Eyes Work When Light Rays Reflect Off an Object and Enter The
How Your Eyes Work When light rays reflect off an object and enter the eyes through the cornea (the transparent outer covering of the eye), you can then see that object. The cornea bends, or refracts, the rays that pass through the round hole of the pupil. The iris (the colored portion of the eye that surrounds the pupil) opens and closes, making the pupil bigger or smaller. This regulates the amount of light passing through. The light rays then pass through the lens, which changes shape so it can further bend the rays and focus them on the retina. The retina, which sits at the back of the eye, is a thin layer of tissue that contains millions of tiny light-sensing nerve cells. These nerve cells are called rods and cones because of their distinct shapes. Cones are concentrated in the center of the retina, in an area called the macula. When there is bright light, cones provide clear, sharp central vision and detect colors and fine details. Rods are located outside the macula and extend all the way to the outer edge of the retina. They provide peripheral or side vision. Rods also allow the eyes to detect motion and help us see in dim light and at night. These cells in the retina convert the light into electrical impulses. The optic nerve sends these impulses to the brain, which produces an image. In the following schematic illustration, click on the names to highlight the parts of the eye. . -

Task-Driven Visual Exploration at the Foveal Scale
Task-driven visual exploration at the foveal scale Natalya Shelchkovaa, Christie Tangb, and Martina Polettia,1 aDepartment of Neuroscience, University of Rochester Medical Center, Rochester, NY 14627; and bDepartment of Psychological and Brain Sciences, Boston University, Boston, MA 02215 Edited by Michael E. Goldberg, Columbia University College of Physicians, New York, NY, and approved January 31, 2019 (received for review July 18, 2018) Humans use saccades to inspect objects of interest with the look at the mouth region (9, 11), whereas scanning the upper foveola, the small region of the retina with highest acuity. This part of the face is mostly associated with recognition tasks (10). process of visual exploration is normally studied over large When the face is presented at an eccentric location, the first scenes. However, in everyday tasks, the stimulus within the saccade to the face is the most important for facial recogni- foveola is complex, and the need for visual exploration may tion (12). It normally brings the gaze close to the nose, but its extend to this smaller scale. We have previously shown that exact landing location is also biased by the task demands (12). fixational eye movements, in particular microsaccades, play an Therefore, by examining saccade landing positions and the scan important role in fine spatial vision. Here, we investigate whether paths of observers looking at faces it is possible to infer the task task-driven visual exploration occurs during the fixation pauses performed (13). Crucially, while these patterns of visual explo- in between large saccades. Observers judged the expression of ration are seen in most subjects, there are significant individual faces covering approximately 1◦, as if viewed from a distance of variations (13–15).