Interference of Scalp and Skull with External Measurements of Brain Isotope Content: Part 1
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lung Transplantation with the OCS (Organ Care System)
Lung Transplantation with the OCSTM (Organ Care System) Lung System Bringing Breathing Lung Preservation to Transplant Patients A Guide for You and Your Family DRAFT ABOUT THIS BOOKLET This booklet was created for patients like you who have been diagnosed with end-stage lung failure and are candidates for a lung transplant. It contains information that will help you and your family learn about options available to you for a transplant. This booklet includes information on your lungs, how they function, and respiratory failure. In addition, you will learn about a new way to preserve lungs before transplantation, called breathing lung preservation. Your doctor is the best person to explain your treatment options and their risks and to help you decide which option is right for you. The booklet explains: • Breathing lung preservation with the OCS™ Lung System • How the OCS™ Lung System works • Who is eligible for the OCS™ Lung System • Lung transplant complications • How the lungs function • What is respiratory failure and the treatment options • What to expect during your treatment • Summary of clinical data for the OCS™ Lung System • Contact Information Please read this booklet carefully and share it with your family and caregivers. For your convenience, a glossary is provided in the front of this booklet. Terms in the text in bold italics are explained in the glossary. If you have questions about the OCS™ Lung System that are not answered in this booklet, please ask your physician. This booklet is intended for general information only. It is not intended to tell you everything you need to know about a lung transplant. -

Case Report AJNT
Arab Journal of Nephrology and Transplantation. 2011 Sep;4(3):155-8 Case Report AJNT High Ureteric Injury Following Multiorgan Recovery: Successful Kidney Transplant with Boari Flap Ureterocystostomy Reconstruction Michael Charlesworth*, Gabriele Marangoni, Niaz Ahmad Department of Transplantation, Division of Surgery, St James’s University Hospital, Leeds, United Kingdom Abstract Keywords: Kidney; Transplant; Ureter; Donor efficiency Introduction: Despite increased utilization of marginal organs, there is still a marked disparity between organ The authors declared no conflict of interest supply and demand for transplantation. To maximize resources, it is imperative that procured organs are in Introduction good condition. Surgical damage at organ recovery can happen and organs are sometimes discarded as a result. Despite the extension of the donor pool with the inclusion We describe a damaged recovered kidney with high of marginal organs and the use of organs donated after ureteric transection that was successfully transplanted cardiac death, there is still a great disparity between the using a primary Boari flap ureterocystostomy. number of patients on the transplant waiting list and the number of kidney transplants performed each year. Case report: The donor kidney was procured form a It is therefore of paramount importance to maximize deceased donor and sustained damage by transection our scarce resources and avoid the discard of otherwise of the ureter just distal to the pelvi-ureteric junction at functional kidneys due to iatrogenic injuries at the time organ recovery. The recipient had been on the transplant of multi-organ recovery. Essentially, three types of organ waiting list for eight years and not accepting this kidney damage can potentially occur: vascular, parenchymal would have seriously jeopardized her chance of future and ureteric. -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Skin Is Not the Largest Organ
View metadata, citation and similar papers at core.ac.uk brought to you by CORE providedRD by Elsevier Sontheimer - Publisher Connector Skin is Not the Largest Organ 3 content. CHS, JNB, and MAS were involved in Paris, France; Division of Genetics and susceptibility to psoriasis vulgaris. J Invest study supervision. Molecular Medicine, St John’s Institute of Dermatol 134:271–3 Dermatology, Guy’s Hospital, London, UK; Martin MA, Klein TE, Dong BJ et al. (2012) Clinical 1,8 4 Alexander A. Navarini , Guy’s and St Thomas’ NHS Foundation pharmacogenetics implementation consortium Trust, Skin Therapy Research Unit, St John’s Laurence Valeyrie-Allanore2,8, guidelines for HLA-B genotype and abacavir 1 Institute of Dermatology, St Thomas’ Hospital, dosing. Clin Pharmacol Ther 91:734–8 Niovi Setta-Kaffetzi , London, UK; 5King’s College Hospital, London, Jonathan N. Barker1,3,4, UK; 6University Medical Center Freiburg, Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N et al. (2013) Rare variations in IL36RN in 1 5 Institute of Medical Biometry and Medical Francesca Capon , Daniel Creamer , severe adverse drug reactions manifesting as 2 6 Informatics, Freiburg, Germany and Jean-Claude Roujeau , Peggy Sekula , 7 acute generalized exanthematous pustulosis. 1 Department of Dermatology, J Invest Dermatol 133:1904–7 Michael A. Simpson , Dokumentationszentrum Schwerer 1 Richard C. Trembath , Hautreaktionen (dZh), Universita¨ts-Hautklinik, Setta-Kaffetzi N, Navarini AA, Patel VM et al. (2013) Maja Mockenhaupt7,8 and Freiburg, Germany Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated 1,3,4,8 8 Catherine H. Smith These authors contributed equally to this work. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

The Neurocranium Forms the Cranial Cavity That Surrounds and Protects the Brain and Brainstem. the Neurocranium Is Formed from T
Name: Wokoma Olobo Benebo Department: Medical Laboratory Science Course: ANA 208 Matric Number: 18/MHS06/055 Assignment 1. Discuss the differences between viscerocranium and neurocranium The neurocranium forms the cranial cavity that surrounds and protects the brain and brainstem. The neurocranium is formed from the occipital bone, two temporal bones, two parietal bones, the sphenoid, ethmoid and frontal bones; they are all joined together with sutures. The viscerocranium bones form the anterior and lower regions of the skull and include the mandible, which attaches through the only truly motile joint found in the skull. The facial skeleton contains the vomer, two nasal conchae, two nasal bones, two maxilla, the mandible, two palatine bones, two zygomatic bones, and two lacrimal bones. 2. Femoral triangle is a special area of the thigh, Discuss Answers: The femoral triangle is a wedge-shaped area formed by a depression between the muscles of the thigh. It is located on the medial aspect of the proximal thigh. It is the region of the passage of the main blood vessels between the pelvis and the lower limb, as well as a large nerve supplying the thigh. It has several Borders and Contents. BORDERS: a. Lateral Border b. Medial Border c. Superior Border CONTENTS: a. Femoral Artery b. Femoral Vein c. Femoral Nerve d. Femoral Canal e. Lymphatics 3. Describe all the muscles of the lower limb that participates during 1/metre social distancing at the period of Covid 19. Answers: a. Rectus Femoris b. Vastus Medialis c. Vastus Lateralis d. Sartorius e. Gracilis f. The Hamstrings g.The Iliopsoas in the hips h. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Study on Asterion and Presence of Sutural Bones in South Indian Dry Skull
Mohammed Ahad et al /J. Pharm. Sci. & Res. Vol. 7(6), 2015, 390-392 Study on Asterion and Presence of Sutural Bones in South Indian Dry Skull Mohammed Ahad(1),Thenmozhi M.S.(2) 1)BDS 1st year, 2) HOD of Anatomy, Saveetha dental college and hospitals Abstract: Aim: To study morphological features of asterion and presence of sutural bones in posterior side of the 25 human skull. Objective: To know the detailed anatomical knowledge of sutural morphology of asterion and formation of sutural bone. Background: Asterion is the point on Norma lateralis where parietal, temporal and occipital bones meet. It has many neurosurgical importance so any variation during surgery cause damage to dural venous sinuses. Presence of sutural bones will complicate surgical orientation, so it is important to study about the formation of sutural bones and its pattern. Materials and methods: The study will be performed on 25 south Indian dry skull of unknown age and sex taken from the department of anatomy at Saveetha dental college and hospital ,Chennai. Reason: A Research on this topic will lead to the outcome of asterion position from various anatomical landmarks and incidence of sutural bone at posterior side of the skull. Keywords: asterion, sutural bones, surgical importance. INTRODUCTION: The asterion is the junction of the parietal, temporal and occipital bone. It is the surgical landmark to the transverse sinus location, which is of great importance in the surgical approaches to the posterior cranial fossa[1].The sutural morphology was classified into two types: Type 1 where a sutural bone was present and Type 2 where sutural bone was absent. -
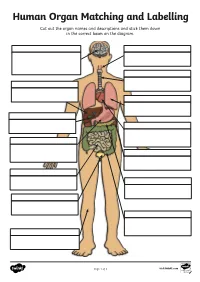
Human Organ Matching and Labelling Cut out the Organ Names and Descriptions and Stick Them Down in the Correct Boxes on the Diagram
Human Organ Matching and Labelling Cut out the organ names and descriptions and stick them down in the correct boxes on the diagram. Page 1 of 3 visit twinkl.com Maintains body temperature using Receives food from the oesophagus sweat and goosebumps. and begins to break it down with digestive juices (enzymes). Controls all of our necessary bodily functions, sends the Transports air from the nose and impulses which allow us to move mouth to the lungs. and enables you to think and learn. Pumps oxygenated blood around your body and receives de- oxygenated blood back. Filters water and salt out of your oesophagus blood and creates urine. bladder Makes bile for digestion, filters out toxins and regulates blood sugar. liver Produces enzymes necessary for large intestine digestion. gall bladder Digests food using enzymes and absorbs nutrients for the blood. kidneys Continues the digestion process, stomach absorbs as much water as possible and expels excess fibre and waste. heart Stores and concentrates bile pancreas produced by the liver. lungs Takes in oxygen, which reaches the blood via the heart. small intestine Stores urine so that we can decide trachea when we want to go to the toilet. skin Transports food and drink from the mouth to the stomach. brain Page 2 of 3 visit twinkl.com Answers brain oesophagus Controls all of our necessary bodily Transports food and drink from the functions, sends the impulses which mouth to the stomach. allow us to move and enables you to think and learn. trachea Transports air from the nose and liver mouth to the lungs. -

Study of Craniometric Point As a Landmark in Performing Posterolateral Surgeries on Skull
Recent Advances in Pathology & Laboratory Medicine Volume 5, Issue 3 - 2019, Pg. No. 17-19 Peer Reviewed & Open Access Journal Research Article Study of Craniometric Point as a Landmark in Performing Posterolateral Surgeries on Skull Sachin Patil1, Dharmendra Kumar2 1Assistant Professor, Department of Anatomy, ANIIMS, Port Blair, Andaman and Nicobar Islands, India. 2Associate Professor & Head, Department of Physical Medicine and Rehabilitation, ANIIMS, Port Blair, Andaman and Nicobar Islands, India. DOI: https://doi.org/10.24321/2454.8642.201917 INFO ABSTRACT Corresponding Author: Introduction: The asterion is craniometric point on the lateral side Dharmendra Kumar, Department of Anatomy, of skull. Importance of asterion lies in that it is primary landmark in ANIIMS, Port Blair, Andaman and Nicobar Islands, performing posterolateral surgeries on skull. India. Material and Methods: In 100 adult dry skulls measurements were E-mail Id: taken on right and left sides of the skull using digital Vernier callipers. [email protected] Two parameters were noted: Distance of the asterion to the root of Orcid Id: zygoma and to the tip of the mastoid process. https://orcid.org/0000-0001-9722-5107 How to cite this article: Result: The mean distance of the asterion to the root of zygoma on Patil S, Kumar D. Study of Craniometric Point as a right side was 56.15+2.40 mm and on left side was 57.48+2.68 mm. The Landmark in Performing Posterolateral Surgeries mean distance of the asterion to the tip of the mastoid process on the on Skull. Rec Adv Path Lab Med 2019; 5(3): 17-19.