Specialty Pharmacy Program Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Phenylbutyrate (PB), Is a New Active Substance
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Ammonpas. This scientific discussion has been updated until 1 November 2001. For information on changes after this date please refer to module 8B. 1. Introduction Ammonaps, sodium phenylbutyrate (PB), is a new active substance. It is indicated as adjunctive therapy in the chronic management of urea cycle disorders, involving deficiencies of carbamylphosphate synthetase, ornithine transcarbamylase, or argininosuccinate synthetase. It is indicated in all patients with neonatal-onset presentation (complete enzyme deficiencies, presenting within the first 28 days of life). It is also indicated in patients with late-onset disease (partial enzyme deficiencies, presenting after the first month of life) who have a history of hyperammonaemic encephalopathy. The dossier submitted in support of the application comprises data generated by the applicant: all chemical/pharmaceutical data, the two mutagenicity studies for Part III, and for Part IV, the bioequivalence study and a review of the US IND/NDA programme. Additional information was available from published literature. Urea cycle disorders (UCD) are inherited deficiencies of one of the enzymes involved in the urea cycle, by which ammonium is converted to urea. Ammonium is highly toxic to nerve cells and hyperammonaemia may result in metabolic derangement, leading to anorexia, lethargy, confusion, coma, brain damage, and death. The most severe forms of UCDs occur early in life (complete enzyme deficiencies). The classic neonatal presentation of all the UCD (with the exception of arginase deficiency) is quite uniform and includes, after a short symptom-free interval of one to five days, poor feeding, vomiting, lethargy, muscular hypotonia, hyperventilation, irritability and convulsions. -
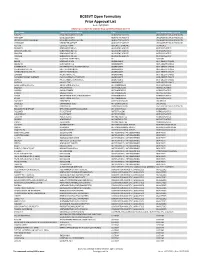
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

2018-2019 Targeted Medication Safety Best Practices for Hospitals
2018-2019 Targeted Medication Safety Best Practices for Hospitals The purpose of the Targeted Medication Safety Best Practices for Hospitals is to identify, inspire, and mobilize widespread, national adoption of consensus-based best practices for specific medication safety issues that continue to cause fatal and harmful errors in patients, despite repeated warnings in ISMP publications. Hospitals can focus their medication safety efforts over the next 2 years on these best practices, which are realistic and have been successfully adopted by numerous organizations. While targeted for the hospital-based setting, some best practices may be applicable to other healthcare settings. The Targeted Medication Safety Best Practices for Hospitals have been reviewed by an external expert advisory panel and approved by the ISMP Board of Trustees. Related issues of the ISMP Medication Safety Alert! are referenced after each best practice. ISMP encourages hospitals that have not implemented the 2016-2017 Targeted Medication Safety Best Practices for Hospitals to do so as a priority, while implementing the 2018-2019 best practices. Organizations need to focus on previous best practices 2, 3, 9 and 11 since these have the lowest implementation rate. Two of the 2016-2017 Targeted Medication Safety Best Practices for Hospitals (number 4 and 7) have been revised for 2018-2019. Best practices number 12 through 14 are new for 2018-2019. www.ismp.org BEST PRACTICE 1: Dispense vinCRIStine (and other vinca alkaloids) in a minibag of a compatible solution and not in a syringe. Rationale: Related ISMP Medication The goal of this best practice is to ensure that vinca alkaloids are Safety Alerts!: administered by the intravenous route only. -

WHO Drug Information Vol 22, No
WHO Drug Information Vol 22, No. 1, 2008 World Health Organization WHO Drug Information Contents Challenges in Biotherapeutics Miglustat: withdrawal by manufacturer 21 Regulatory pathways for biosimilar Voluntary withdrawal of clobutinol cough products 3 syrup 22 Pharmacovigilance Focus Current Topics WHO Programme for International Drug Proposed harmonized requirements: Monitoring: annual meeting 6 licensing vaccines in the Americas 23 Sixteen types of counterfeit artesunate Safety and Efficacy Issues circulating in South-east Asia 24 Eastern Mediterranean Ministers tackle Recall of heparin products extended 10 high medicines prices 24 Contaminated heparin products recalled 10 DacartTM development terminated and LapdapTM recalled 11 ATC/DDD Classification Varenicline and suicide attempts 11 ATC/DDD Classification (temporary) 26 Norelgestromin-ethynil estradiol: infarction ATC/DDD Classification (final) 28 and thromboembolism 12 Emerging cardiovascular concerns with Consultation Document rosiglitazone 12 Disclosure of transdermal patches 13 International Pharmacopoeia Statement on safety of HPV vaccine 13 Cycloserine 30 IVIG: myocardial infarction, stroke and Cycloserine capsules 33 thrombosis 14 Erythropoietins: lower haemoglobin levels 15 Recent Publications, Erythropoietin-stimulating agents 15 Pregabalin: hypersensitivity reactions 16 Information and Events Cefepime: increased mortality? 16 Assessing the quality of herbal medicines: Mycophenolic acid: pregnancy loss and contaminants and residues 36 congenital malformation 17 Launch -

European Medicines Agency Accepts Marketing Authorization Application for Asfotase Alfa As a Treatment for Patients with Hypophosphatasia
July 24, 2014 European Medicines Agency Accepts Marketing Authorization Application for Asfotase Alfa as a Treatment for Patients with Hypophosphatasia -- Application designated for review under accelerated assessment process -- CHESHIRE, Conn.--(BUSINESS WIRE)-- Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) today announced that the Marketing Authorization Application (MAA) for asfotase alfa, an investigational, first-in-class targeted enzyme replacement therapy for the treatment of hypophosphatasia (HPP), has been validated and granted accelerated assessment by the European Medicines Agency (EMA). The acceptance of this MAA marks the beginning of the review process in the European Union (EU) for this potential new treatment. "HPP is a devastating disease for patients and their families due to progressive deterioration of bones and muscle weakness, which can result in impaired respiratory function, severe disability and death," said Leonard Bell, M.D., Chief Executive Officer of Alexion. "If approved, asfotase alfa would be the first therapy for patients with this life-threatening disorder." The EU filing includes positive data from 68 patients with pediatric-onset HPP (ranging from newborns to 66 years of age) enrolled in three pivotal prospective studies and their extensions, as well as a retrospective natural history study in infants. In April, Alexion initiated the rolling submission of a Biologics License Application (BLA) for asfotase alfa as a treatment for patients with HPP with the U.S. Food and Drug Administration (FDA). About -

Drug Consumption in 2017 - 2020
Page 1 Drug consumption in 2017 - 2020 2020 2019 2018 2017 DDD/ DDD/ DDD/ DDD/ 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital ATC code Subgroup or chemical substance day % day % day % day % A ALIMENTARY TRACT AND METABOLISM 322,79 3 312,53 4 303,08 4 298,95 4 A01 STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01A STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01AA Caries prophylactic agents 11,90 3 10,48 4 8,42 5 8,45 7 A01AA01 sodium fluoride 11,90 3 10,48 4 8,42 5 8,45 7 A01AA03 olaflur 0,00 - 0,00 - 0,00 - 0,00 - A01AB Antiinfectives for local oral treatment 2,36 8 2,31 7 2,31 7 2,02 7 A01AB03 chlorhexidine 2,02 6 2,10 7 2,09 7 1,78 7 A01AB11 various 0,33 21 0,21 0 0,22 0 0,24 0 A01AD Other agents for local oral treatment 0,02 0 0,03 0 0,04 0 - - A01AD02 benzydamine 0,02 0 0,03 0 0,04 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 73,05 3 71,13 3 69,32 3 68,35 3 A02A ANTACIDS 2,23 1 2,22 1 2,20 1 2,30 1 A02AA Magnesium compounds 0,07 22 0,07 22 0,08 22 0,10 19 A02AA04 magnesium hydroxide 0,07 22 0,07 22 0,08 22 0,10 19 A02AD Combinations and complexes of aluminium, 2,17 0 2,15 0 2,12 0 2,20 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 2,17 0 2,15 0 2,12 0 2,20 0 A02B DRUGS FOR PEPTIC ULCER AND 70,82 3 68,91 3 67,12 3 66,05 4 GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 0,17 7 0,74 4 1,10 4 1,11 5 A02BA02 ranitidine 0,00 1 0,63 3 0,99 3 0,99 4 A02BA03 famotidine 0,16 7 0,11 8 0,11 10 0,12 9 A02BB Prostaglandins 0,04 62 -

Asfotase Alfa for Infants and Young Children with Hypophosphatasia: 7 Year Outcomes of a Single-Arm, Open-Label, Phase 2 Extension Trial
Articles Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial Michael P Whyte, Jill H Simmons, Scott Moseley, Kenji P Fujita, Nicholas Bishop, Nada J Salman, John Taylor, Dawn Phillips, Mairead McGinn, William H McAlister Summary Background Our previous phase 2, open-label study of 11 infants and young children with life-threatening perinatal or Lancet Diabetes Endocrinol infantile hypophosphatasia showed 1 year safety and efficacy of asfotase alfa, an enzyme replacement therapy. We 2019; 7: 93–105 aimed to report the long-term outcomes over approximately 7 years of treatment. Published Online December 14, 2018 http://dx.doi.org/10.1016/ Methods We did a prespecified, end of study, 7 year follow-up of our single-arm, open-label, phase 2 trial in which S2213-8587(18)30307-3 children aged 3 years or younger with life-threatening perinatal or infantile hypophosphatasia were recruited from This online publication has been ten hospitals (six in the USA, two in the UK, one in Canada, and one in the United Arab Emirates). Patients received corrected. The corrected version asfotase alfa (1 mg/kg three times per week subcutaneously, adjusted to 3 mg/kg three times per week if required) for first appeared at thelancet. up to 7 years (primary treatment period plus extension phase) or until the product became commercially available; com/diabetes-endocrinology on January 22, 2019 dosage adjustments were made at each visit according to changes in the patient’s weight. The primary objectives of See Comment page 76 this extension study were to assess the long-term tolerability of asfotase alfa, defined as the number of patients with Center for Metabolic Bone one or more treatment-emergent adverse events, and skeletal manifestations associated with hypophosphatasia, Disease and Molecular evaluated using the Radiographic Global Impression of Change (RGI-C) scale (−3 indicating severe worsening, and Research, Shriners Hospital for +3 complete or near-complete healing). -

Horizon Therapeutics Public Annual Report 2020
Horizon Therapeutics Public Annual Report 2020 Form 10-K (NASDAQ:HZNP) Published: February 26th, 2020 PDF generated by stocklight.com octb inte UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 001-35238 HORIZON THERAPEUTICS PUBLIC LIMITED COMPANY (Exact name of Registrant as specified in its charter) Ireland Not Applicable (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) Connaught House, 1st Floor 1 Burlington Road, Dublin 4, D04 C5Y6, Ireland Not Applicable (Address of principal executive offices) (Zip Code) 011 353 1 772 2100 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol Name of Each Exchange on Which Registered Ordinary shares, nominal value $0.0001 per share HZNP The Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐. Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒. Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

The Effectiveness of Correcting Abnormal Metabolic Profiles
Received: 25 April 2019 Revised: 17 June 2019 Accepted: 19 June 2019 DOI: 10.1002/jimd.12139 ORIGINAL ARTICLE The effectiveness of correcting abnormal metabolic profiles Peter Theodore Clayton UCL Great Ormond Street Institute of Child Health, London, UK Abstract Inborn errors of metabolism cause disease because of accumulation of a metabolite Correspondence before the blocked step or deficiency of an essential metabolite downstream of the Peter Clayton, UCL Great Ormond Street Institute of Child Health, London, UK. block. Treatments can be directed at reducing the levels of a toxic metabolite or Email: [email protected] correcting a metabolite deficiency. Many disorders have been treated successfully first in a single patient because we can measure the metabolites and adjust treat- Communicating Editor: Sander M. Houten ment to get them as close as possible to the normal range. Examples are drawn from Komrower's description of treatment of homocystinuria and the author's trials 5 of treatment in bile acid synthesis disorders (3β-hydroxy-Δ -C27-steroid dehydro- genase deficiency and Δ4-3-oxosteroid 5β-reductase deficiency), neurotransmitter amine disorders (aromatic L-amino acid decarboxylase [AADC] and tyrosine hydroxylase deficiencies), and vitamin B6 disorders (pyridox(am)ine phosphate oxidase deficiency and pyridoxine-dependent epilepsy [ALDH7A1 deficiency]). Sometimes follow-up shows there are milder and more severe forms of the disease and even variable clinical manifestations but by measuring the metabolites we can adjust the treatment to get the metabolites into the normal range. Biochemical mea- surements are not subject to placebo effects and will also show if the disorder is improving spontaneously. -

Asfotase Alfa (Strensiq) for Treatment of Hypophosphatasia in Infants and Children
AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 08: Functional Limitations and Disability Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA290-2010-00006-C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 December 2015 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290-2010-00006-C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. This report’s content should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual topic profiles are developed for technologies and programs that appear to be close to diffusion into practice in the United States. Those reports are sent to various experts with clinical, health systems, health administration, and/or research backgrounds for comment and opinions about potential for impact. The comments and opinions received are then considered and synthesized by ECRI Institute to identify interventions that experts deemed, through the comment process, to have potential for high impact. Please see the methods section for more details about this process. -

L-Citrulline
L‐Citrulline Pharmacy Compounding Advisory Committee Meeting November 20, 2017 Susan Johnson, PharmD, PhD Associate Director Office of Drug Evaluation IV Office of New Drugs L‐Citrulline Review Team Ben Zhang, PhD, ORISE Fellow, OPQ Ruby Mehta, MD, Medical Officer, DGIEP, OND Kathleen Donohue, MD, Medical Officer, DGIEP, OND Tamal Chakraborti, PhD, Pharmacologist, DGIEP, OND Sushanta Chakder, PhD, Supervisory Pharmacologist, DGIEP, OND Jonathan Jarow, MD, Advisor, Office of the Center Director, CDER Susan Johnson, PharmD, PhD, Associate Director, ODE IV, OND Elizabeth Hankla, PharmD, Consumer Safety Officer, OUDLC, OC www.fda.gov 2 Nomination • L‐citrulline has been nominated for inclusion on the list of bulk drug substances for use in compounding under section 503A of the Federal Food, Drug and Cosmetic Act (FD&C Act) • It is proposed for oral use in the treatment of urea cycle disorders (UCDs) www.fda.gov 3 Physical and Chemical Characterization • Non‐essential amino acid, used in the human body in the L‐form • Well characterized substance • Soluble in water • Likely to be stable under ordinary storage conditions as solid or liquid oral dosage forms www.fda.gov 4 Physical and Chemical Characterization (2) • Possible synthetic routes – L‐citrulline is mainly produced by fermentation of L‐arginine as the substrate with special microorganisms such as the L‐arginine auxotrophs arthrobacterpa rafneus and Bacillus subtilis. – L‐citrulline can also be obtained through chemical synthesis. The synthetic route is shown in the scheme below. This -

Transaction Drug 1St (DIN) 2Nd (PIN) 3Rd (PIN) 4Th (PIN) 5Th (PIN) 6Th
Transaction Drug 1st (DIN) 2nd (PIN) 3rd (PIN) 4th (PIN) 5th (PIN) 6th (PIN) 7th (PIN) 8th (PIN) 9th (PIN) 10th (PIN) 11th (PIN) 12th (PIN) 13th (PIN) Alectinib (Alecensaro®) 02458136 00904400 − − − − − − − − − − − 150 mg capsule Alemtuzumab (LemtradaTM) 02418320 00904161 00904162 00904163 00904164 00904165 00904166 00904167 − − − − − 12 mg / 1.2 mL single-use vial Asfotase alfa (Strensiq®) 02444615 00904483 00904484 00904485 − − − − − − − − − 18 mg / 0.45 mL single-use vial Asfotase alfa (Strensiq®) 02444623 00904486 00904487 00904488 00904489 00904490 − − − − − − − 28 mg / 0.7 mL single-use vial Asfotase alfa (Strensiq®) 02444631 00904491 00904492 00904493 − − − − − − − − − 40 mg / 1 mL single-use vial Asfotase alfa (Strensiq®) 02444658 00904494 00904495 00904496 00904497 00904498 00904499 00904500 00904501 00904502 00904504 00904505 − 80 mg / 0.8 mL single-use vial Canakinumab (Ilaris®) 150 mg/mL powder for solution 02344939 00904404 00903809 00904410 − − − − − − − − − for injection Canakinumab (Ilaris®) 02460351 00904405 00904411 00904412 − − − − − − − − − 150 mg/mL solution for injection Ceftolozane / Tazobactam 02446901 00904433 − − − − − − − − − − − (Zerbaxa®) 1 g / 0.5 g vial Cerliponase Alfa (Brineura®) 150 mg / 5 mL solution for 02484013 00904634 00904635 00904636 − − − − − − − − − intracerebroventricular infusion Cladribine (MavencladTM) 02470179 00904524 00904525 00904526 00904642 − − − − − − − − 10 mg tablet Cysteamine (ProcysbiTM) 02464713 00904354 00904355 − − − − − − − − − − 75 mg delayed-release capsule Daclastavir (DaklinzaTM)