Evolution and Development in Cave Animals: from Fish to Crustaceans" (2012)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
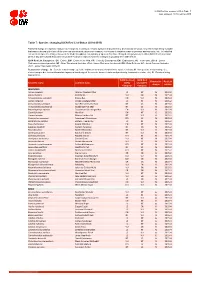
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Hotspots of Subterranean Biodiversity in Caves and Wells
...J Oklahoma State Univ. Interlibrary Loan Call#: pdf w Location: pdf a:: Journal Title: Journal of cave and karst studies ; <( the National Speleological Society bulletin. Volume: 62 Issue: 1 ARIEL MonthNear: 2000 MaxCost: $501FM Pages: 11-17 Scanned by: Article Title: DC Culver and B Sket; Hotspots of Subterranean Shipped by: Biodiversity in Caves and Wells Ariel: 128.194.84.50 Article Author: Fax: 979-458-2032 or Borrower: TXA Shipping Address: Patron: Bandel, Micaela TAMU Libraries - College Station .. TAE z 41 HOU I- ILL Number: 85855670 Lending ~ String: *OKS,COD,IXA,TXH,VA@ ""0 The work from which this copy was made did not include a formal copyright notice. Copyright law may protect this work. Uses may be al lowed with permission from the rights holder, or if the copyright on the work has expired, or if the use is "fair use" or if it is within another exemption. The user of this work is responsible for determining its lawful uses. David C. Culver and Boris Sket - Hotspots of Subterranean Biodiversity in Caves and Wells . .Journal of Cave and Karst Studies 62(1):11-17. HOTSPOTS OF SUBTERRANEAN BIODIVERSITY IN CAVES AND WELLS DAVID C. CULVER Department of Biology, American University, 4400 Massachusetts Ave., NW, Washington, DC 20016, USA, [email protected] BORIS SKET Department of Biology, Biotechnical Faculty, University of Ljubljana, PO. Box 2995, 1001 Ljubljana, SLOVENIA, [email protected] We documented 18 caves and two karst wells that have 20 or more stygobites and troglobites. Crustacea dominated the aquatic fauna. Taxonomic composition ofthe terrestrial fauna varied, but Arachnida and Insecta together usually dominated. -

Monitoring Methodology and Protocols for 20 Habitats, 20 Species and 20 Birds
1 Finnish Environment Institute SYKE, Finland Monitoring methodology and protocols for 20 habitats, 20 species and 20 birds Twinning Project MK 13 IPA EN 02 17 Strengthening the capacities for effective implementation of the acquis in the field of nature protection Report D 3.1. - 1. 7.11.2019 Funded by the European Union The Ministry of Environment and Physical Planning, Department of Nature, Republic of North Macedonia Metsähallitus (Parks and Wildlife Finland), Finland The State Service for Protected Areas (SSPA), Lithuania 2 This project is funded by the European Union This document has been produced with the financial support of the European Union. Its contents are the sole responsibility of the Twinning Project MK 13 IPA EN 02 17 and and do not necessarily reflect the views of the European Union 3 Table of Contents 1. Introduction .......................................................................................................................................................... 6 Summary 6 Overview 8 Establishment of Natura 2000 network and the process of site selection .............................................................. 9 Preparation of reference lists for the species and habitats ..................................................................................... 9 Needs for data .......................................................................................................................................................... 9 Protocols for the monitoring of birds .................................................................................................................... -

Belgian Register of Marine Species
BELGIAN REGISTER OF MARINE SPECIES September 2010 Belgian Register of Marine Species – September 2010 BELGIAN REGISTER OF MARINE SPECIES, COMPILED AND VALIDATED BY THE VLIZ BELGIAN MARINE SPECIES CONSORTIUM VLIZ SPECIAL PUBLICATION 46 SUGGESTED CITATION Leen Vandepitte, Wim Decock & Jan Mees (eds) (2010). Belgian Register of Marine Species, compiled and validated by the VLIZ Belgian Marine Species Consortium. VLIZ Special Publication, 46. Vlaams Instituut voor de Zee (VLIZ): Oostende, Belgium. 78 pp. ISBN 978‐90‐812900‐8‐1. CONTACT INFORMATION Flanders Marine Institute – VLIZ InnovOcean site Wandelaarkaai 7 8400 Oostende Belgium Phone: ++32‐(0)59‐34 21 30 Fax: ++32‐(0)59‐34 21 31 E‐mail: [email protected] or [email protected] ‐ 2 ‐ Belgian Register of Marine Species – September 2010 Content Introduction ......................................................................................................................................... ‐ 5 ‐ Used terminology and definitions ....................................................................................................... ‐ 7 ‐ Belgian Register of Marine Species in numbers .................................................................................. ‐ 9 ‐ Belgian Register of Marine Species ................................................................................................... ‐ 12 ‐ BACTERIA ............................................................................................................................................. ‐ 12 ‐ PROTOZOA ........................................................................................................................................... -

Monograph of the Cyprinid Fis~Hes of the Genus Garra Hamilton (173)
MONOGRAPH OF THE CYPRINID FIS~HES OF THE GENUS GARRA HAMILTON By A. G. K. MENON, Zoologist, ,Zoological Surt1ey of India, Oalcutta. (With 1 Table, 29 Text-figs. and 6 Plates) CONTENTS Page I-Introduction 175 II-Purpose and general results 176 III-Methods and approaches 176 (a) The definition of Measurements 176 (b) The analysis of Intergradation 178 (c) The recognition of subspecies. 179 (d) Procedures in the paper 180 (e) Evaluation of systematic characters 181 (I) Abbreviations of names of Institutions 181 IV-Historical sketch 182 V-Definition of the genus 187 VI-Systematic section 188 (a) The variabilis group 188 (i) The variabilis Complex 188 1. G. variabilis 188 2. G. rossica 189 (b) The tibanica group 191 (i) The tibanica Complex 191 3. G. tibanica. 191 4. G. quadrimaculata 192 5. G. ignestii 195 6. G. ornata 196 7. G. trewavasi 198 8. G. makiensis 198 9. G. dembeensis 199 10. G. ethelwynnae 202 (ii) The rufa complex 203 11. G. rufa rufa 203 12. G. rufa obtusa 205 13. O. barteimiae 206 (iii) The lamta complex 208 14. G. lamta 208 15. G. mullya 212 16. G. 'ceylonensis ceylonensis 216 17. G. c. phillipsi 216 18. G. annandalei 217 (173) 174 page (iv) The lissorkynckus complex 219 19. G. lissorkynchus 219 20. G. rupecula 220 ~ (v) The taeniata complex 221 21. G. taeniata. 221 22" G. borneensis 224 (vi) The yunnanensis complex 224 23. G. yunnanensis 225 24. G. gracilis 229 25. G. naganensis 226 26. G. kempii 227 27. G. mcOlellandi 228 28. G. -

(Peracarida: Isopoda) Inferred from 18S Rdna and 16S Rdna Genes
76 (1): 1 – 30 14.5.2018 © Senckenberg Gesellschaft für Naturforschung, 2018. Relationships of the Sphaeromatidae genera (Peracarida: Isopoda) inferred from 18S rDNA and 16S rDNA genes Regina Wetzer *, 1, Niel L. Bruce 2 & Marcos Pérez-Losada 3, 4, 5 1 Research and Collections, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007 USA; Regina Wetzer * [[email protected]] — 2 Museum of Tropical Queensland, 70–102 Flinders Street, Townsville, 4810 Australia; Water Research Group, Unit for Environmental Sciences and Management, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa; Niel L. Bruce [[email protected]] — 3 Computation Biology Institute, Milken Institute School of Public Health, The George Washington University, Ashburn, VA 20148, USA; Marcos Pérez-Losada [mlosada @gwu.edu] — 4 CIBIO-InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, 4485-661 Vairão, Portugal — 5 Department of Invertebrate Zoology, US National Museum of Natural History, Smithsonian Institution, Washington, DC 20013, USA — * Corresponding author Accepted 13.x.2017. Published online at www.senckenberg.de/arthropod-systematics on 30.iv.2018. Editors in charge: Stefan Richter & Klaus-Dieter Klass Abstract. The Sphaeromatidae has 100 genera and close to 700 species with a worldwide distribution. Most are abundant primarily in shallow (< 200 m) marine communities, but extend to 1.400 m, and are occasionally present in permanent freshwater habitats. They play an important role as prey for epibenthic fishes and are commensals and scavengers. Sphaeromatids’ impressive exploitation of diverse habitats, in combination with diversity in female life history strategies and elaborate male combat structures, has resulted in extraordinary levels of homoplasy. -

Characidae) from Different Hydrographic Basins: Analysis of Agnors, CMA3 and 18S Rdna
Karyotype diversity of four species of the incertae sedis group (Characidae) from different hydrographic basins: analysis of AgNORs, CMA3 and 18S rDNA M.M. Mendes, R. da Rosa, L. Giuliano-Caetano and A.L. Dias Departamento de Biologia Geral, Centro de Ciências Biológicas, Universidade Estadual de Londrina, Londrina, PR, Brasil Corresponding author: A.L. Dias E-mail: [email protected] Genet. Mol. Res. 10 (4): 3596-3608 (2011) Received March 21, 2011 Accepted August 31, 2011 Published November 22, 2011 DOI http://dx.doi.org/10.4238/2011.November.22.5 ABSTRACT. A large number of genera in the tropical fish family Characidae are incertae sedis. Cytogenetic analysis was made of four of these species: Astyanax eigenmanniorum, Deuterodon stigmaturus, Hyphessobrycon luetkenii, and H. anisitsi, collected from various hy- drographic basins: hydrographic system from Laguna dos Patos/RS, Tramandaí basin/RS and Tibagi River basin/PR. The first two species were collected in their type locality in the State of Rio Grande do Sul. The 2n = 48 karyotype was observed only in A. eigenmanniorum, while the other species had 2n = 50 chromosomes, with different karyotypic formulas. There was weak heterochromatin staining in the pericentro- meric region of A. eigenmanniorum, D. stigmaturus and H. luetkenni chromosomes. In H. anisitsi, heterochromatin appeared to be more abundant and distributed in the pericentromeric and terminal regions of the chromosomes; three pairs showed more evident heterochromatic blocks. There were multiple Ag-NORs in all populations, visualized by FISH with an 18S rDNA probe. While D. stigmaturus and H. luetkenii Genetics and Molecular Research 10 (4): 3596-3608 (2011) ©FUNPEC-RP www.funpecrp.com.br Karyotype diversity of an incertae sedis group in Characidae 3597 had conserved AgNOR, CMA3 and 18S rDNA sites, the other two spe- cies showed intra- and interindividual variation at these sites. -

Bibliography of Astyanax Cavefishes
Bibliography of Astyanax Cavefishes William R. Elliott, Association for Mexican Cave Studies Readers may send additions and corrections to me at [email protected] 804 references listed by authors, 11/22/2017 Aguayo-Camargo, J.E. 1998. The middle Cretaceous El Abra Limestone at its type locality (facies, diagenesis and oil emplacement), east-central Mexico. Revista Mexicana de Ciencias Geológicas 1998, 15:1–8. Albert, Richard O. 2006. The Great Sierra de El Abra Expedition. AMCS Activities Newsletter, 29:132-143. Albert, Richard O. 2016. The Search for Sótano del Grunge: Exploration of Sótano del Malpaís. AMCS Activities Newsletter, 40:96-101. Albert, Richard O. 2018. The Second Great Sierra de El Abra Expedition. Unpublished manuscript. AMCS., in press. 100 p. Alexander, Ed. 1965. Trip report. AMCS Newsletter, 1:116. Alexander, Ed. 1965. Trip report. AMCS Newsletter, 1:52-54. Alunni A., Menuet A., Candal E., Pénigault JB., Jeffery W.R., Rétaux S. 2007. Developmental mechanisms for retinal degeneration in the blind cavefish Astyanax mexicanus. Journal of Comparative Neurology. 2007 Nov 10; 505(2):221- 33. Alvarado, Carlos Garita, 2017. Parallel evolution of body shape in Astyanax (Characidae) morphotype. AIM 2017 posters:47. Álvarez, José 1959. Nota preliminar sobre la ictiofauna del estado de San Luís Potosí. Act. Cientif. Potosina,3(1):71-88. Álvarez, José. 1946. Revision del genero Anoptichthys con descripción de una especie nueva (Pisces, Characidae). Annales de la Escuela Nacional de Ciencias Biológicas de Mexico, 4:263-282. Álvarez, José. 1947. Descripción de Anoptichthys hubbsi caracínido ciego de la cueva de los Sabinos, S.L.P. -

Crustacea of the Environs of St. John, New Brunswick, Canada
Proceedings of the Iowa Academy of Science Volume 74 Annual Issue Article 38 1967 Crustacea of the Environs of St. John, New Brunswick, Canada Richard W. Coleman Upper Iowa College Let us know how access to this document benefits ouy Copyright ©1967 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Coleman, Richard W. (1967) "Crustacea of the Environs of St. John, New Brunswick, Canada," Proceedings of the Iowa Academy of Science, 74(1), 240-246. Available at: https://scholarworks.uni.edu/pias/vol74/iss1/38 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Coleman: Crustacea of the Environs of St. John, New Brunswick, Canada Crustacea of the Environs of St. John, New Brunswick,. Canada RICHARD w. COLEMAN 1 Abstract. The following species of crustacea were col lected in a survey of the environs of St. John, New Bruns wick, Canada: Crangonyx gracilis Smith, Gammarus duebeni Lilly, Gammarus lawrencianus Bousfield, Gammarus ocean icus Segerstrale, Gammarus tigrinus Sexton, Hyale nilssoni Rathke, Hyalella azteca Sauss., ]aera albifrons Leach, Mar inogammarus finmarchicus Dahl, Marinogammarus obtusatus Dahl and Marinogammarus stoerensis Reid: Hyalella azteca was the predominant species in purely fresh water lakes; Gammarus Ugrinus predominated in fresh to brackish waters; and Hyale nilssoni appeared to be the dominant species from marine sources. This discussion is based upon a paper entitled "A Report to the Provincial Department of Public Health, Province of New Brunswick, Fredericton, New Brunswick, Canada, on a Survey for Certain Crustacea of the Environs of St. -

Hotspots of Subterranean Biodiversity in Caves and Wells
David C. Culver and Boris Sket - Hotspots of Subterranean Biodiversity in Caves and Wells. Journal of Cave and Karst Studies 62(1):11-17. HOTSPOTS OF SUBTERRANEAN BIODIVERSITY IN CAVES AND WELLS DAVID C. CULVER Department of Biology, American University, 4400 Massachusetts Ave., NW, Washington, DC 20016, USA, [email protected] BORIS SKET Department of Biology, Biotechnical Faculty, University of Ljubljana, P.O. Box 2995, 1001 Ljubljana, SLOVENIA, [email protected] We documented 18 caves and two karst wells that have 20 or more stygobites and troglobites. Crustacea dominated the aquatic fauna. Taxonomic composition of the terrestrial fauna varied, but Arachnida and Insecta together usually dominated. Geographically, the sites were concentrated in the Dinaric Karst (6 caves). Sites tended to have high primary productivity or rich organic input from the surface, be large caves, or have permanent groundwater (phreatic water). Dokumentirala sva 18 jam in dva kraška vodnjaka, iz katerih je znanih 20 ali vec vrst troglobiontov in stigobiontov. V vodni favni preladujejo raki. Taksonomski sestav kopenske favne je raznolik, vendar pajkovci in zuzelke skupaj navadno prevladujejo. Najvec takšnih jam (šest) je v Dinarskem krasu. Nadpovprecno so zastopane jame z lastno primarno produkcijo ali bogatim vnosom hrane s površja, obsezne jame in jame, ki vkljucujejo tudi freatsko plast. Over the past few years, there has been a growing aware- terns at individual sites. Even though most subterranean bio- ness and concern with biodiversity worldwide. Books and diversity results from the accumulation of different species monographs with a focus on biodiversity have appeared (e.g., from nearby sites, there are some outstanding examples of high Wilson 1992; Master et al. -

Some Malacostracan Crustacean Assemblages in the Southern and Western Baltic Sea
Rostock. Meeresbiolog. Beitr. (2001) 9, 127-143 Michael L. ZETTLER Some malacostracan crustacean assemblages in the southern and western Baltic Sea Runnning head: Crustacean assemblages in the Baltic Sea Abstract The paper presents a survey of four crustacean benthic assemblages in the southern and western Baltic Sea. Relative abundance and a multivariate method (cluster analysis) were used to describe these assemblages in German Baltic wa- ters. This study is based mainly on 17 sampling events carried out during 1998/99. The salinity ranged between 2 and 22 psu and the depth varied between 0.4 and 47.3 m. The habitats were silty sands with Mytilus-aggregates, the silt zone below 20 m, shallow stone and boulder grounds and lagoons and fjord-like bays. For as- sessment of sediment structure, current, patchiness and larger crustaceans (e.g. Crangon crangon, Carcinus maenas) in the deeper parts an underwater video- camera was used which was mounted on a sledge. In total 43 species were recor- ded. The most common and important species were Diastylis rathkei, Gammarus salinus, G. zaddachi, Gastrosaccus spinifer, Pontoporeia femorata, Calliopius lae- viusculus, Corophium insidiosum, Leptocheirus pilosus, Jaera albifrons s.l. and Sphaeroma hookeri. One habitat (Kleines Haff, Oder estuary) is characterised through some non-indigenous species, such as Pontogammarus robustoides, Gammarus tigrinus and Corophium curvispinum, especially. Kurzfassung Die Studie präsentiert einen Untersuchung über 4 benthische Krebsgemein- schaften in der südlichen und westlichen Ostsee. Mit relativen Abundanzen und der Cluster-Analyse werden diese Gemeinschaften beschrieben. Die Studie basiert hauptsächlich auf 17 Beprobungen zwischen 1998 und 1999. Die Salinität rangierte zwischen 2 und 22 psu und die Wassertiefe lag zwischen 0,4 und 47,3 m. -

Oxyeleotris Colasi (Teleostei: Eleotridae), a New Blind Cave Fish from Lengguru in West Papua, Indonesia
Oxyeleotris colasi (Teleostei: Eleotridae), a new blind cave fish from Lengguru in West Papua, Indonesia by Laurent POUYAUD* (1), KADARUSMAN (1, 2), Renny K. HADIATY (3), Jacques SLEMBROUCK (1), Napoleon LEMAUK (4), Ruby V. KUSUMAH (5) & Philippe KEITH (6) ABSTRACT. - Oxyeleotris colasi is the first hypogean fish recorded from West Papua. The habitat consists of a freshwater pool in the cave of Jabuenggara located in the heart of Seraran anticline in the limestone karst of Lengguru. The new spe- cies is most closely related to the blind cave fishO. caeca described by Allen (1996) from eastern New Guinea. The two troglomorphic species are hypothesised to be related to O. fimbriata, an epigean freshwater gudgeon that ranges widely in New Guinea and northern Australia (Allen, 1996). Oxyeleotris colasi differs from its congeners by the absence of eyes, its skin and fins being totally depigmented, the presence of a well developed sensory papillae system partly consisting of low raised fleshy ridges on each side of the head, a reduced number of cephalic sensory pores, a reduced number of scales on head and body, a long head with a short snout length, a narrow mouth width and a long upper jaw length, body shape with a shallow anterior body depth and narrow body width, a long and deep caudal peduncle, long predorsal and prepectoral lengths, and a long pectoral fin. RÉSUMÉ. - Oxyeleotris colasi, une nouvelle espèce de poisson cavernicole de Lengguru en Papouasie occidentale (Teleostei : Eleotridae). Oxyeleotris colasi est la première espèce de poisson hypogée décrite de Papouasie occidentale. Elle a été capturée dans un trou d’eau douce situé dans la grotte de Jabuenggara au cœur de l’anticlinal de Seraran dans le karst de Lengguru.