Vascular-Derived SPARC and Serpine1 Regulate Interneuron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Migration in the Developing Rodent Olfactory System
Cell. Mol. Life Sci. (2016) 73:2467–2490 DOI 10.1007/s00018-016-2172-7 Cellular and Molecular Life Sciences REVIEW Cell migration in the developing rodent olfactory system 1,2 1 Dhananjay Huilgol • Shubha Tole Received: 16 August 2015 / Revised: 8 February 2016 / Accepted: 1 March 2016 / Published online: 18 March 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract The components of the nervous system are Abbreviations assembled in development by the process of cell migration. AEP Anterior entopeduncular area Although the principles of cell migration are conserved AH Anterior hypothalamic nucleus throughout the brain, different subsystems may predomi- AOB Accessory olfactory bulb nantly utilize specific migratory mechanisms, or may aAOB Anterior division, accessory olfactory bulb display unusual features during migration. Examining these pAOB Posterior division, accessory olfactory bulb subsystems offers not only the potential for insights into AON Anterior olfactory nucleus the development of the system, but may also help in aSVZ Anterior sub-ventricular zone understanding disorders arising from aberrant cell migra- BAOT Bed nucleus of accessory olfactory tract tion. The olfactory system is an ancient sensory circuit that BST Bed nucleus of stria terminalis is essential for the survival and reproduction of a species. BSTL Bed nucleus of stria terminalis, lateral The organization of this circuit displays many evolution- division arily conserved features in vertebrates, including molecular BSTM Bed nucleus of stria terminalis, medial mechanisms and complex migratory pathways. In this division review, we describe the elaborate migrations that populate BSTMa Bed nucleus of stria terminalis, medial each component of the olfactory system in rodents and division, anterior portion compare them with those described in the well-studied BSTMpl Bed nucleus of stria terminalis, medial neocortex. -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Ganglionic Eminence: Anatomy and Pathology in Fetal MRI Eminencia Ganglionar: Anatomía Y Patología En Resonancia Magnética Fetal
case report Ganglionic Eminence: Anatomy and Pathology in Fetal MRI Eminencia ganglionar: Anatomía y patología en resonancia magnética fetal Daniel Martín Rodríguez1 Manuel Recio Rodríguez2 Pilar Martínez Ten3 María Nieves Iglesia Chaves4 Summary Key words (MeSH) We present two cases of fetal MRI where anomalies of the ganglionic eminences (GE) are detected, one case in a single pregnancy and another in a twin gestation with only one of the affected fetuses. Cavitation Alterations in the ganglionic eminences are rare entities, with very few published cases, both by Magnetic resonance MRI and fetal ultrasound, which are usually associated with severe neurological alterations. The imaging MR findings of the pathology of the GE in these two cases are described. These findings were not Embryonic development visible on the previous ultrasound. Resumen Palabras clave (DeCS) Se presentan dos casos de resonancia magnética (RM) fetal en los que se detectan anomalías de Cavitación las eminencias ganglionares (EG): un caso en una gestación única y otro en una gestación gemelar Imagen por resonancia con solo uno de los fetos afectado. Las alteraciones en las eminencias ganglionares son entidades poco frecuentes, con muy pocos casos publicados, tanto por RM como por ecografía fetal, que magnética suelen asociarse con alteraciones neurológicas graves. Se describen los hallazgos por RM de la Desarrollo embrionario patología de las EG en estos dos casos, no visibles en la ecografía previa. Introduction cavitations and C-shaped morphology, without evidence Ganglionic eminences (GE) are transient, prolifera- of bleeding. No intermediate neuronal layer was identi- tive, embryonic structures of the ventral telencephalon, fied between the germinal matrix and the immature outer which are located on the lateral wall of the frontal cortex, but a prominent germinal matrix was identified. -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Redalyc.Normal Neuronal Migration
Salud Mental ISSN: 0185-3325 [email protected] Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz México Flores Cruz, María Guadalupe; Escobar, Alfonso Normal neuronal migration Salud Mental, vol. 34, núm. 1, enero-febrero, 2011, pp. 61-66 Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=58220040008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Salud Mental 2011;34:61-66 Normal neuronal migration Normal neuronal migration María Guadalupe Flores Cruz,1 Alfonso Escobar1 Artículo original SUMMARY with cytoplasmic dilatation, and then the centrosome and Golgi apparatus approach it, finally nucleus advances to the cytoplasmic Ontogenesis of both central and peripheral nervous systems depends dilatation. Movement of centrosome and nucleus depends on integrity on basic, molecular and cellular mechanisms of the normal neuronal of a microtubule network. Most of the microtubules surrounding the migration. Any deviation leads to neural malformations. All neural nucleus are tyrosinated, making them dynamic; microtubules at the cells and structures derive from the neural ectoderm, which under the anterior pole of the nucleus, near the centrosome, are acetylated. influence of the notochord and the molecules Noggin and Chordin, is Once neurons reach their final destination, they need to cancel transformed consecutively into neural plate, neural groove, neural tube the migratory program and differentiate. The mechanisms are and primary vesicles. -
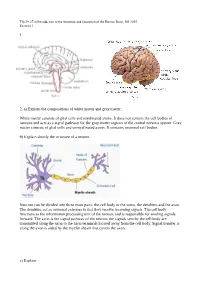
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons
The Journal of Neuroscience, September 1989, g(9): 30303039 Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons Gilles Laurent and Malcolm Burrows Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, England The gain of local reflexes of one leg of a locust can be altered partmentalized, with synaptic inputs and their associated by mechanosensory inputs generated by movements of or conductance changes restricted to particular branches. In tactile inputs to an adjacent leg. Touching the mesothoracic this way, an individual nonspiking neuron could contribute tarsus, for example, increases the number of spikes that are simultaneously to several local circuits. The inputs from dif- produced by the metathoracic slow extensor tibiae motor ferent intersegmental interneurons could then modulate these neuron and enhances the depolarization of flexor tibiae mo- pathways independently. tor neuron in response to imposed movements of the chor- dotonal organ in the ipsilateral hind femur. The sensory in- Nonspiking interneurons in the metathoracic ganglion of the formation from the middle leg is conveyed directly to locust receive direct inputs from mechanosensory afferents on nonspiking interneurons and motor neurons controlling the one hindleg and are essential elements in local reflex movements movements of the hindleg by a population of mesothoracic of that leg (Laurent and Burrows, 1988; Burrows et al., 1988). intersegmental interneurons (Laurent and Burrows, 1989). They also receive direct inputs from intersegmental intemeu- The metathoracic nonspiking interneurons receive direct in- rons that process the mechanosensory inputs from an ipsilateral puts from receptors on a hindleg and are, therefore, a point middle leg (Laurent and Burrows, 1989). -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles. -

Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells
Journal of Developmental Biology Review Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells Nickesha C. Anderson *, Christopher Y. Chen and Laura Grabel Department of Biology, Wesleyan University, 52 Lawn Avenue, Middletown, CT 06459, USA; [email protected] (C.Y.C.); [email protected] (L.G.) * Correspondence: [email protected]; Tel.: +1-860-778-8898 Academic Editors: Henk Roelink and Simon J. Conway Received: 11 July 2016; Accepted: 17 August 2016; Published: 26 August 2016 Abstract: Loss or damage of cortical inhibitory interneurons characterizes a number of neurological disorders. There is therefore a great deal of interest in learning how to generate these neurons from a pluripotent stem cell source so they can be used for cell replacement therapies or for in vitro drug testing. To design a directed differentiation protocol, a number of groups have used the information gained in the last 15 years detailing the conditions that promote interneuron progenitor differentiation in the ventral telencephalon during embryogenesis. The use of Hedgehog peptides and agonists is featured prominently in these approaches. We review here the data documenting a role for Hedgehog in specifying interneurons in both the embryonic brain during development and in vitro during the directed differentiation of pluripotent stem cells. Keywords: Sonic hedgehog; GABAergic interneurons; medial ganglionic eminence; pluripotent stem cells 1. Introduction Glutamatergic projection neurons and gamma-aminobutyric acid-containing (GABAergic) inhibitory interneurons are the two major classes of neurons in the cerebral cortex. Despite constituting only around 20%–30% of the total neuron population in the mammalian cortex, inhibitory interneurons play a key role in modulating the overall activity of this region [1]. -

Retinal Inputs Signal Astrocytes to Recruit Interneurons Into Visual Thalamus
Retinal inputs signal astrocytes to recruit interneurons into visual thalamus Jianmin Sua,1, Naomi E. Charalambakisb,1, Ubadah Sabbagha,c, Rachana D. Somaiyaa,c, Aboozar Monavarfeshania,d, William Guidob,2, and Michael A. Foxa,d,e,2 aCenter for Neurobiology Research, Fralin Biomedical Research Institute at Virginia Tech Carilion, Roanoke, VA 24016; bDepartment of Anatomical Sciences and Neurobiology, University of Louisville School of Medicine, Louisville, KY 40202; cGraduate Program in Translational Biology, Medicine, and Health, Virginia Tech, Blacksburg, VA 24061; dDepartment of Biological Sciences, Virginia Tech, Blacksburg, VA 24061; and eDepartment of Pediatrics, Virginia Tech Carilion School of Medicine, Roanoke, VA 24016 Edited by Carol Ann Mason, Columbia University, New York, NY, and approved December 24, 2019 (received for review July 29, 2019) Inhibitory interneurons comprise a fraction of the total neurons in collection of cell types based on their distribution, gene and the visual thalamus but are essential for sharpening receptive field neurochemical expression, and projection patterns (5–7, 25). properties and improving contrast-gain of retinogeniculate trans- Despite the presence of GABAergic interneurons in the visual mission. During early development, these interneurons undergo thalamus of the adult mouse, these cells are largely absent at long-range migration from germinal zones, a process regulated by birth when RGC axons innervate these nuclei (12). Also lacking the innervation of the visual thalamus by retinal ganglion cells. from the visual thalamus at these early ages are nonretinal inputs Here, using transcriptomic approaches, we identified a motogenic that arise from the neocortex, brainstem, and the thalamic re- cue, fibroblast growth factor 15 (FGF15), whose expression in the ticular nucleus.