Reimbursement Efforts at Nass Areas of Focus for Nass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MISSED? Metastatic Spinal Cord Compression NA Quraishi, C Esler ∗ BMJ 342 (7805), 1023-1025
PUBLICATIONS (ABSTRACTS EXCLUDED) 2014: Metastatic spinal cord compression as a result of the unknown primary tumour. Quraishi NA, Ramoutar D, Sureshkumar D, Manoharan SR, Spencer A, Arealis G, Edwards KL, Boszczyk BM. Eur Spine J. 2014 Apr 2. Trans-oral approach for the management of a C2 neuroblastoma. Salem KM, Visser J, Quraishi NA. Eur Spine J. 2014 Feb 19. Calcified giant thoracic disc herniations: considerations and treatment strategies. Quraishi NA, Khurana A, Tsegaye MM, Boszczyk BM, Mehdian SM. Eur Spine J. 2014 Apr;23 Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Varga PP, Szövérfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, Chou D, Qurais NA, Reynolds JJ, Luzzati A, Williams R, Fehlings MG, Germscheid NM, Lazary A, Rhines LD. Eur Spine J. 2014 Dec 23. Expert's comment concerning Grand Rounds case entitled: "trans-oral approach for the management of a C2 neuroblastoma. (K. M. I. Salem, J. Visser, and N. A. Quraishi).Choi D. Eur Spine J. 2015 Jan;24(1):177-9. Diagnosis and treatment of a rectal-cutaneous fistula: a rare complication of coccygectomy. Behrbalk E, Uri O, Maxwell-Armstrong C, Quraishi NA. Eur Spine J. 2014 Nov 1. A cohort study to evaluate cardiovascular risk of selective and nonselective cyclooxygenase inhibitors (COX-Is) in arthritic patients attending orthopedic department of a tertiary care hospital. Bhosale UA, Quraishi N, Yegnanarayan R, Devasthale D. Niger Med J. 2014 Sep;55(5):417-22. An evidence-based medicine model for rare and often neglected neoplastic conditions. Fisher CG, Goldschlager T, Boriani S, Varga PP, Rhines LD, Fehlings MG, Luzzati A, Dekutoski MB, Reynolds JJ, Chou D, Berven SH, Williams RP, Quraishi NA, Bettegowda C, Gokaslan ZL. -

CMM-314: Hip Surgery-Arthroscopic and Open Procedures Version 1.0.2019
CLINICAL GUIDELINES CMM-314: Hip Surgery-Arthroscopic and Open Procedures Version 1.0.2019 Clinical guidelines for medical necessity review of speech therapy services. © 2019 eviCore healthcare. All rights reserved. Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-314: Hip Surgery-Arthroscopic and Open Procedures CMM-314.1: Definitions 3 CMM-314.2: General Guidelines 4 CMM-314.3: Indications and Non-Indications 4 CMM-314.4 Experimental, Investigational, or Unproven 6 CMM-314.5: Procedure (CPT®) Codes 7 CMM-314.6: References 10 © 2019 eviCore healthcare. All rights reserved. Page 2 of 13 400 Buckwalter Place Boulevard, Bluffton, SC 29910 • (800) 918-8924 www.eviCore.com Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-314.1: Definitions Femoroacetabular Impingement (FAI) is an anatomical mismatch between the head of the femur and the acetabulum resulting in compression of the labrum or articular cartilage during flexion. The mismatch can arise from subtle morphologic alterations in the anatomy or orientation of the ball-and-socket components (for example, a bony prominence at the head-neck junction or acetabular over-coverage) with articular cartilage damage initially occurring from abutment of the femoral neck against the acetabular rim, typically at the anterosui per or aspect of the acetabulum. Although hip joints can possess the morphologic features of FAI without symptoms, FAI may become pathologic with repetitive movement and/or increased force on the hip joint. High-demand activities may also result in pathologic impingement in hips with normal morphology. s It ha been proposed that impingement with damage to the labrum and/or acetabulum is a causative factor in the development of hip osteoarthritis, and that as many as half of cases currently categorized as primary osteoarthritis may have an etiology of FAI. -

Physicians As Assistants at Surgery: 2016 Update
Physicians as Assistants at Surgery: 2016 Update Participating Organizations: American College of Surgeons American Academy of Ophthalmology American Academy of Orthopaedic Surgeons American Academy of Otolaryngology – Head and Neck Surgery American Association of Neurological Surgeons American Pediatric Surgical Association American Society of Colon and Rectal Surgeons American Society of Plastic Surgeons American Society of Transplant Surgeons American Urological Association Congress of Neurological Surgeons Society for Surgical Oncology Society for Vascular Surgery Society of American Gastrointestinal Endoscopic Surgeons The American College of Obstetricians and Gynecologists The Society of Thoracic Surgeons Physicians as Assistants at Surgery: 2016 Update INTRODUCTION This is the seventh edition of Physicians as Assistants at Surgery, a study first undertaken in 1994 by the American College of Surgeons and other surgical specialty organizations. The study reviews all procedures listed in the “Surgery” section of the 2016 American Medical Association’s Current Procedural Terminology (CPT TM). Each organization was asked to review new codes since 2013 that are applicable to their specialty and determine whether the operation requires the use of a physician as an assistant at surgery: (1) almost always; (2) almost never; or (3) some of the time. The results of this study are presented in the accompanying report, which is in a table format. This table presents information about the need for a physician as an assistant at surgery. Also, please note that an indication that a physician would “almost never” be needed to assist at surgery for some procedures does NOT imply that a physician is never needed. The decision to request that a physician assist at surgery remains the responsibility of the primary surgeon and, when necessary, should be a payable service. -
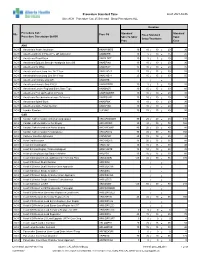
Standard Times Report
Procedure Standard Time as of: 2021-04-06 Site: ACH Procedure Cat: 25 Selected Show Procedures: ALL Duration Procedure Cat / Standard Standard Site Proc Cd Fixed Standard Procedure Description Std BK Skin To Skin/ Total Setup/Teardown Proc Case ANA ACH Anesthesia Awake Intubation ANAWKINTB 10 10 / 10 = 20 30 ACH Anesthesia Block in PACU Pre OR Admission ANABKRR 10 5 / 10 = 15 25 ACH Anesthesia Blood Patch ANABLDPT 10 5 / 5 = 10 20 ACH Anesthesia Epidural Steroid+/-Analgesia Inject SS ANAEPINJ 10 10 / 10 = 20 30 ACH Anesthesia for XRay ANAXRAY 15 10 / 10 = 20 35 ACH Anesthesia Insert Long Line IV<1 Year ANALINE<1 25 15 / 15 = 30 55 ACH Anesthesia Insert Long Line IV>1 Year ANALINE>1 25 15 / 15 = 30 55 ACH Anesthesia Intubate Only OR ANAINTB / = ACH Anesthesia Intubate Only PACU ANAINTBRR 10 5 / 5 = 10 20 ACH Anesthesia Local+/-Regional Block State Type ANABKOR 30 15 / 15 = 30 60 ACH Anesthesia Post Op Readmit to PACU ANARADMRR 10 10 / 10 = 20 30 ACH Anesthesia Pseudocholinesterase Deficiency ANAPSURR 10 10 / 10 = 20 30 ACH Anesthesia Spinal Block ANASPBK 10 10 / 10 = 20 30 ACH Anesthesia Spine Facet Injection ANASPINJ 10 10 / 10 = 20 30 ACH Lumbar Puncture LUPUNC 10 25 / 15 = 40 50 CAR ACH Cardiac Catheterization & Balloon Angioplasty HRCARCBANG 90 20 / 20 = 40 130 ACH Cardiac Catheterization w Full Biopsy HRCARCBX 90 40 / 30 = 70 160 ACH Cardiac Catheterization w Partial Biopsy HRCARCBXP 40 20 / 20 = 40 80 ACH Cardiac Catheterization+/-Valvoplasty HRCARCVL 90 40 / 30 = 70 160 ACH Catheter Insertion Aphoresis CAINAPHP 20 15 / 10 = 25 45 -

Decellularized Tissue Engineered Constructs Using Cell Sheet Technology
Decellularized Tissue Engineered Constructs Using Cell Sheet Technology Author Farag, Amro Ahmed Mahmoud Published 2016 Thesis Type Thesis (PhD Doctorate) School School of Dentistry and Oral Health DOI https://doi.org/10.25904/1912/2310 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/367720 Griffith Research Online https://research-repository.griffith.edu.au Decellularized Tissue engineered constructs using cell sheet technology Amro Ahmed Mahmoud Farag BDSc, M.Sc. (Periodontics) School of Dentistry Griffith Health Griffith University Submitted in fulfilment of the requirements of the degree of Doctor of Philosophy 3/30/2016 I Statement of Originality I, Amro Farag declare that this work has not previously been submitted for a degree or diploma in any university. To the best of my knowledge and belief, the thesis contains no material previously published or written by another person except where due reference is made in the thesis itself. (Signed)_____________________________ Amro Farag II Contents Statement of Originality ......................................................................................... II Contents ............................................................................................................... III List of Tables ..................................................................................................... VIII List of Figures..................................................................................................... -

682-8137 Or [email protected] NASS Releases Must-Have
FOR IMMEDIATE RELEASE Contact: Nicolle Heller, NASS October 7, 2015 (773) 682-8137 or [email protected] NASS Releases Must-Have Tool for Spine Specialists: eBook of Coverage Policy Recommendations --Subscription is Free to All NASS Members-- Burr Ridge, IL—In a game-changing move for patients and spine specialists, the North American Spine Society (NASS) unveiled its latest resource, the NASS Coverage Policy Recommendations eBook. An annual subscription to this online book of NASS coverage guidance on common spine treatments is free to all NASS members and available for purchase by non-members. “Spine specialists spend a great deal of time—time they could be spending caring for their patients—doing labor-intensive research, filling out onerous paperwork and participating in peer-to-peer reviews to justify even common treatments to payers,” said Christopher M. Bono, MD, NASS First Vice-President and the first Coverage Committee Chair. “This essential eBook of evidence-based recommendations will help them navigate this system and get their patients the care they need to quickly get back to enjoying active, productive lives.” NASS Coverage Policy Recommendations is a 100+ page online, searchable book that includes NASS’ current 17 coverage recommendations, extensive citations, active links to published research and interactive elements such as video. NASS will add recommendations and revise existing recommendations periodically based on the availability of new evidence-based literature and the feedback it receives from members, patients and insurance entities. Subscribers automatically will have access to the most recent edition online. To develop each published recommendation, the multi-disciplinary NASS Coverage Committee conducts an extensive review of available scientific literature. -

Products of Ambulatory Surgery 2008 Procedure Codes
Products of Ambulatory Surgery 2008 Procedure Codes CPT Begin /HCPCS CPT Description PAS PAS Description Date 28035 Release, tarsal tunnel (posterior tibial nerve decompression) 1 Nerve Repair 2/1/2004 28080 Excision, interdigital (Morton) neuroma, single, each 1 Nerve Repair 2/1/2004 61790 Creation of lesion by stereotactic method, percutaneous, by neurolytic agent (eg, alcohol, thermal, electrical, radiofrequency); gasserian ganglion 1 Nerve Repair 2/1/2004 Insertion or replacement of cranial neurostimulator pulse generator or receiver, direct or inductive coupling; with connection to a single electrode 61885 array 1 Nerve Repair 2/1/2004 InIncisioncision aandnd subcutasubcutaneousneous pplacementlacement ooff ccranialranial nneurostimulatoreurostimulator pupulselse gegeneratornerator oorr rreceiver,eceiver, ddirectirect oorr ininductiveductive coupcoupling;ling; wiwithth coconnectionnnection to ttwowo oorr 61886 more electrode arrays 1 Nerve Repair 2/1/2004 62268 Percutaneous aspiration, spinal cord cyst or syrinx 1 Nerve Repair 2/1/2004 62269 Biopsy of spinal cord, percutaneous needle 1 Nerve Repair 2/1/2004 62270 Spinal puncture, lumbar, diagnostic 1 Nerve Repair 2/1/2004 62272 Spinal puncture, therapeutic, for drainage of cerebrospinal fluid (by needle or catheter) 1 Nerve Repair 2/1/2004 62273 Injection, epidural, of blood or clot patch 1 Nerve Repair 2/1/2004 62280 Injection/infusion of neurolytic substance (eg, alcohol, phenol, iced saline solutions), with or without other therapeutic substance; subarachnoid 1 Nerve Repair 2/1/2004 -

SJH Procedures
SJH Procedures - Orthopedics and Podiatry Service New Name Old Name CPT Code Service ABLATION, PLANTAR WART, USING CO2 LASER LASER VAPORIZATION (WARTS/LESIONS) PLANTAR FOOT W CO2 LASER 17110 Destruction (eg, laser surgery, electrosurgery, cryosurgery, Podiatry chemosurgery, surgical curettement), of benign lesions other than skin tags or cutaneous vascular proliferative lesions; up to 14 lesions ACROMIOPLASTY, ARTHROSCOPIC, WITH DISTAL CLAVICLE EXCISION ARTHROSCOPY DISTAL CLAVICLE ACROMIOPLASTY/REPAIR/EXCISION 29824 Arthroscopy, shoulder, surgical; distal claviculectomy including Orthopedics distal articular surface (Mumford procedure) 29826 Arthroscopy, shoulder, surgical; decompression of subacromial Orthopedics space with partial acromioplasty, with coracoacromial ligament (ie, arch) release, when performed (List separately in addition to code for primary procedure) ALLOGRAFT, OSTEOCHONDRAL, KNEE, OPEN OSTEOCHONDRAL ALLOGRAFT TRANSPLANTATION OPEN KNEE 27415 Osteochondral allograft, knee, open Orthopedics AMPUTATION, BELOW KNEE AMPUTATION LEG BELOW KNEE *27880 Amputation, leg, through tibia and fibula; Vascular, Orthopedics *27881 Amputation, leg, through tibia and fibula; with immediate Vascular, Orthopedics fitting technique including application of first cast *27882 Amputation, leg, through tibia and fibula; open, circular Vascular, Orthopedics (guillotine) *27886 Amputation, leg, through tibia and fibula; re-amputation Vascular, Orthopedics AMPUTATION, FINGER AMPUTATION FINGER 26910 Amputation, metacarpal, with finger or thumb (ray -
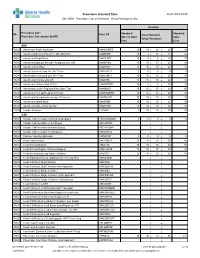
RGH Procedure Cat: 25 Selected Show Procedures: ALL
Procedure Standard Time as of: 2021-04-06 Site: RGH Procedure Cat: 25 Selected Show Procedures: ALL Duration Procedure Cat / Standard Standard Site Proc Cd Fixed Standard Procedure Description Std BK Skin To Skin/ Total Setup/Teardown Proc Case ANA RGH Anesthesia Awake Intubation ANAWKINTB 10 10 / 10 = 20 30 RGH Anesthesia Block in PACU Pre OR Admission ANABKRR 10 5 / 10 = 15 25 RGH Anesthesia Blood Patch ANABLDPT 10 5 / 5 = 10 20 RGH Anesthesia Epidural Steroid+/-Analgesia Inject SS ANAEPINJ 10 10 / 10 = 20 30 RGH Anesthesia for XRay ANAXRAY 15 10 / 10 = 20 35 RGH Anesthesia Insert Long Line IV<1 Year ANALINE<1 0 0 / 0 = 0 0 RGH Anesthesia Insert Long Line IV>1 Year ANALINE>1 10 10 / 10 = 20 30 RGH Anesthesia Intubate Only OR ANAINTB 15 10 / 10 = 20 35 RGH Anesthesia Intubate Only PACU ANAINTBRR 10 5 / 5 = 10 20 RGH Anesthesia Local+/-Regional Block State Type ANABKOR 30 15 / 15 = 30 60 RGH Anesthesia Post Op Readmit to PACU ANARADMRR 10 10 / 10 = 20 30 RGH Anesthesia Pseudocholinesterase Deficiency ANAPSURR 10 10 / 10 = 20 30 RGH Anesthesia Spinal Block ANASPBK 10 10 / 10 = 20 30 RGH Anesthesia Spine Facet Injection ANASPINJ 10 10 / 10 = 20 30 RGH Lumbar Puncture LUPUNC 5 15 / 10 = 25 30 CAR RGH Cardiac Catheterization & Balloon Angioplasty HRCARCBANG 0 0 / 0 = 0 0 RGH Cardiac Catheterization w Full Biopsy HRCARCBX / = RGH Cardiac Catheterization w Partial Biopsy HRCARCBXP / = RGH Cardiac Catheterization+/-Valvoplasty HRCARCVL / = RGH Catheter Insertion Aphoresis CAINAPHP 0 0 / 0 = 0 0 RGH Heart Cardioversion HRCARDVS 15 15 / 15 = 30 -

Resection of the Coccyx As an Outpatient Procedure
Orthopedic Reviews 2020; volume 12:8813 Resection of the coccyx as an treatment and injection therapy have failed outpatient procedure patients can be treated surgically with Correspondence: Ante M. Kalstad, coccygectomy.1,2 Department of Orthopaedic Surgery, St. Traditionally, coccygectomy patients Olav’s University Hospital, 7006 Trondheim, 1-3 Ante M. Kalstad, spend several days in hospital after their Norway. 1 Tel.: +47 90888107 Rainer G. Knobloch, operation. In the 1990s, the average stay 1,3 E-mail: [email protected] Vilhjalmur Finsen after this procedure was 7-10 days.3 1Department of Orthopaedic Surgery, Outpatient surgery (also known as day Key words: Coccyx; tailbone; coccygectomy; St. Olav’s University Hospital, surgery, same-day surgery or ambulatory outpatient surgery; ambulatory surgery. Trondheim; 2Norwegian Armed Forces surgery) refers to surgical procedures that are Funding: None. Joint Medical Services; 3Faculty of performed without staying overnight in the hospital. Following advances in peri- and Medicine, Norwegian University of Conflict of interest: The authors declare no postoperative pain control regimens and Science and Technology, NTNU, conflicts of interest. early rehabilitation protocols there has been Trondheim, Norway a trend in other areas of surgery towards Ethics approval: The study protocol was performing more outpatient procedures. In reviewed by the Regional committee for med- recent years, this has included procedures ical and health research ethics in Central such as unicompartmental knee arthroplasty, Norway (2016/460) who found that it did not Abstract and even total hip arthroplasty,4 commonly need their approval. regarded as an inpatient procedure. This We wished to determine if Availability of data and materials: Data and coccygectomy as an outpatient procedure is development has the benefit of reducing materials are available in the main text. -

Hip Surgery – Arthroscopic and Open Procedures
CLINICAL GUIDELINES CMM-314 ~ Hip Surgery – Arthroscopic and Open Procedures Version 19.0 Effective August 11, 2017 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies:This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2016 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2017 eviCore healthcare. All rights reserved. CMM-314~ Hip Surgery-Arthroscopic and Open Procedures CMM-314.1: Definitions 3 CMM-314.2: General Guidelines 3 CMM-314.3: Indications and Non-Indications 4 CMM-314.4: Procedure (CPT®) Codes 5 CMM-314.5: References 8 Page 2 of 10 CMM-314: Hip Surgery-Arthroscopic and Open Procedures CMM-314.1: Definitions Femoroacetabular Impingement (FAI) is a condition that has been recently recognized, is an anatomical mismatch between the head of the femur and the acetabulum resulting in compression of the labrum or articular cartilage during flexion. The mismatch can arise from subtle morphologic alterations in the anatomy or orientation of the ball-and-socket components (for example, a bony prominence at the head-neck junction or acetabular over coverage) with articular cartilage damage initially occurring from abutment of the femoral neck against the acetabular rim, typically at the anterosuperior aspect of the acetabulum. -
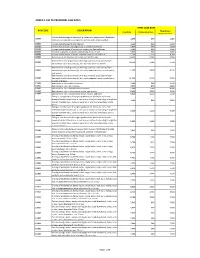
Description Rvs Code Annex 2. List of Procedure Case Rates First Case Rate
ANNEX 2. LIST OF PROCEDURE CASE RATES FIRST CASE RATE RVS CODE DESCRIPTION Health Care Case Rate Professional Fee Institution Fee Incision and drainage of abscess (e.g., carbuncle, suppurative hidradenitis, 10060 3,640 840 2,800 cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia) 10080 Incision and drainage of pilonidal cyst 3,640 840 2,800 10120 Incision and removal of foreign body, subcutaneous tissues 3,640 840 2,800 10140 Incision and drainage of hematoma, seroma, or fluid collection 3,640 840 2,800 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst 3,640 840 2,800 10180 Incision and drainage, complex, postoperative wound infection 5,560 1,260 4,300 11000 Debridement of extensive eczematous or infected skin 10,540 5,040 5,500 Debridement including removal of foreign material associated w/ open 11010 10,540 5,040 5,500 fracture(s) and/or dislocation(s); skin and subcutaneous tissues Debridement including removal of foreign material associated w/ open 11011 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 11,980 5,880 6,100 and muscle Debridement including removal of foreign material associated w/ open 11012 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 12,120 6,720 5,400 muscle, and bone 11040 Debridement; skin, partial thickness 3,640 840 2,800 11041 Debridement; skin, full thickness 3,640 840 2,800 11042 Debridement; skin, and subcutaneous tissue 5,680 1,680 4,000 11043 Debridement; skin, subcutaneous tissue, and muscle 8,020 2,520 5,500 11044 Debridement;