Essential Roles of the Sperm Centrosome in Human Fertilization: Developing the Therapy for Fertilization Failure Due to Sperm Centrosomal Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

When Does Human Life Begin? Christian Thinking and Contemporary Opposition
Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING The substance of this booklet is an extract from The Morning-After Pill – Uncovering the Truth, published by The Christian Institute in 2007: http://www.christian.org.uk/resource/the-morning-after-pill Copyright © The Christian Institute 2017 The author has asserted his right under Section 77 of the Copyright, Designs & Patents Act 1988 to be identified as the author of this work. First printed in June 2011 Reprinted in May 2015 and August 2017 ISBN 978-1-901086-47-8 Published by The Christian Institute Wilberforce House, 4 Park Road, Gosforth Business Park, Newcastle upon Tyne, NE12 8DG All rights reserved No part of this publication may be reproduced, or stored in a retrieval system, or transmitted, in any form or by any means, mechanical, electronic, photocopying, recording or otherwise, without the prior permission of The Christian Institute. All scripture quotations, unless otherwise indicated, are taken from the HOLY BIBLE, NEW INTERNATIONAL VERSION®. NIV®. Copyright © 1973, 1978, 1984 by International Bible Society. Used by permission of Zondervan. All rights reserved. The Christian Institute is a Company Limited by Guarantee, registered in England as a charity. Company No. 263 4440, Charity No. 100 4774. A charity registered in Scotland. Charity No. SC039220 Contents 5 1 . Introduction 7 2 . The answer from the Bible 17 3 . The view of the early church 21 4 . The drift from the biblical worldview 25 5 . -

Chapter 38: Reproduction and Development Computer Test Bank Cryopreservation
Chapter 38 Organizer Reproduction and Development Refer to pages 4T-5T of the Teacher Guide for an explanation of the National Science Education Standards correlations. Teacher Classroom Resources Activities/FeaturesObjectivesSection MastersSection TransparenciesReproducible Reinforcement and Study Guide, pp. 167-168 L2 Section Focus Transparency 93 L1 ELL Section 38.1 1. Identify the parts of the male and Inside Story: Sex Cell Production, p. 1033 Section 38.1 female reproductive systems. Problem-Solving Lab 38-1, p. 1035 Concept Mapping, p. 38 L3 ELL Basic Concepts Transparency 74 L2 ELL Human Reproductive 2. Summarize the negative feedback Human Critical Thinking/Problem Solving, p. 38 L3 Basic Concepts Transparency 75 L2 ELL Systems control of reproductive hormones. Reproductive Content Mastery, pp. 185-186, 188 L1 Reteaching Skills Transparency 55 L1P ELL National Science Education 3. Sequence the stages of the menstrual Systems P P cycle. Standards UCP.1-3, UCP.5; P C.1, C.5; F.1 (2 sessions, 11/ P 2 Reinforcement and Study Guide, p. 169 L2 P Section Focus Transparency 94 L1 ELLP blocks) Section 38.2 P LS BioLab and MiniLab Worksheets, pp. 169-170 L2 Reteaching Skills Transparency 56a,P 56b L1 LS P LS Development Laboratory Manual, pp. 277-284 L2 ELL P Before Birth LS Section 38.2 4. Summarize the events during each MiniLab 38-1: Examining Sperm and Egg Content Mastery, pp. 185,LS 187-188 L1 LS P LS trimester of pregnancy. Attraction, p. 1038 LS Development Before MiniLab 38-2: Making a Graph of Fetal Size, P LS P Reinforcement and Study Guide, p. -

Unit6:Human Reproduction Pregnancy and Embryonic Development Parturition and Lactation
H.S SECOND YEAR Unit6:Human Reproduction Pregnancy and embryonic development Parturition and lactation BY: Dr. LUNA PHUKAN HUMAN FERTILIZATION AND DEVELOPMENT Key Terms Term Meaning Gamete : A reproductive (sex) cell. In males, sperm; in females, eggs Fertilization : The process in sexual reproduction in which a male gamete and female gamete fuse to form a new cell Zygote : Cell resulting from fertilization Diploid (2n) : Cell that contains two sets of homologous chromosomes Haploid (n) : Cell that contains only a single set of genes Apoptosis :The process of programmed cell death Differentiation : The process by which cells become specialized in structure and function Human fertilization and development Fertilization is the process in which haploid gametes fuse to form a diploid cell called a zygote. To ensure that each zygote has the correct number of chromosomes, only one sperm can fuse with one egg. Stages of human development Zygotic stage: The zygote is formed when the male gamete (sperm) and female gamete (egg) fuse. Blastocyst stage: The single-celled zygote begins to divide into a solid ball of cells. Then, it becomes a hollow ball of cells called a blastocyst, attaching to the lining of the mother's uterus. Embryonic stage: The major internal organs and external features begin to emerge, forming an embryo. In this stage, the heart, brain, and spinal cord become visible. Arms and legs start to develop. Fetal stage: Once the formed features of the embryo begin to grow and develop, the organism is considered a fetus. Differentiation and specialization of structures happens during this time. REPRODUCTIVE SYSTEM REVIEW Key terms Term Meaning Gamete: A reproductive (sex) cell. -
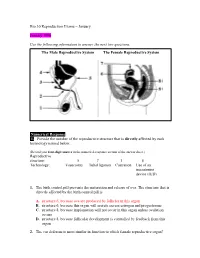
Numerical Response 1. Provide the Number of the Reproductive Structure That Is Directly Affected by Each Technology Named Below
Bio 30 Reproduction Exams – January January 1996 Use the following information to answer the next two questions. The Male Reproductive System The Female Reproductive System Numerical Response 1. Provide the number of the reproductive structure that is directly affected by each technology named below. (Record your four-digit answer in the numerical-response section of the answer sheet.) Reproductive structure: ______5__ ____7____ __1____ ___8_____ Technology: Vasectomy Tubal ligation Castration Use of an intrauterine device (IUD) 1. The birth control pill prevents the maturation and release of ova. The structure that is directly affected by the birth control pill is A. structure 6, because ova are produced by follicles in this organ B. structure 6, because this organ will secrete excess estrogen and progesterone C. structure 8, because implantation will not occur in this organ unless ovulation occurs D. structure 8, because follicular development is controlled by feedback from this organ 2. The vas deferens is most similar in function to which female reproductive organ? A. Ovary B. Uterus C. Vagina D. Fallopian tube Use the following information to answer the next question. Possible Effects of Testosterone 1 Inhibits skeletal muscle development 2 Enhances skeletal muscle development 3 Inhibits development of body hair 4 Promotes development of body hair 5 Inhibits gametogenesis 6 Stimulates gametogenesis 7 Enhances growth of the larynx 8 Inhibits growth of the larynx Numerical Response 2. Select all the correct effects of normal levels of testosterone in an adolescent male. (Record your answer in lowest-to-highest numerical order in the numerical-response section of the answer sheet.) Answer: __2467_____ Use the following information to answer the next two questions. -

Embryology Text Books: Implications for Fetal Research Dianne N
The Linacre Quarterly Volume 61 | Number 2 Article 6 May 1994 "New Age" Embryology Text Books: Implications for Fetal Research Dianne N. Irving Follow this and additional works at: https://epublications.marquette.edu/lnq Recommended Citation Irving, Dianne N. (1994) ""New Age" Embryology Text Books: Implications for Fetal Research," The Linacre Quarterly: Vol. 61 : No. 2 , Article 6. Available at: https://epublications.marquette.edu/lnq/vol61/iss2/6 "New Age" Embryology Text Books: Implications for Fetal Research by Dianne N. Irving, M.A., Ph.D. The author is Assistant Professor, History ofPhilosophy/Bioethies at De Sales School of Theology, Washington, D. C. As outrageous as it is that so much incorrect science has been and still is being used in the scientific, medical and bioethics literature to argue that fetal "personhood" does not arrive until some magical biological marker event during human embryological development, now we can witness the "new wave" consequences of passively allowing such incorrect "new age" science to be published and eventually accepted by professionals and non-professionals alike. Once these scientifically erroneous claims, and the erroneoliS philosophical and theological concepts they engender, are successfully imbedded in these bodies of literature and in our collective consciousnesses, the next logical step is to imbed them in our text books, reference materials and federal regulations. Such is the case with the latest fifth edition of a highly respected embryology text book by Keith Moore - The Developing Human. 1 This text is used in most medical schools and graduate biology departments here, and in many institutions abroad. Several definitions and redefinitions of scientific terms it uses are incorporated, it would seem, in order to support the "new age" political agenda of abortion and fetal research. -

New Insights Into Human Early Embryo Development: a Particular Theoretical Study
14 International Journal of Modern Anthropology Int. J. Mod. Anthrop. (2018) Vol: 2, Issue No: 11, pp: 14 - 46 Available online at: www.ata.org.tn ; DOI: http://dx.doi.org/10.4314/ijma.v2i11.1 Original Synthesis Article New insights into human early embryo development: a particular theoretical study Hassen Chaabani Laboratory of Human Genetics and Anthropology, Faculty of Pharmacy, University of Monastir, 5000 Monastir, Tunisia. E.mail: [email protected] (Received 18 January 2018; Accepted 5 Mars 2018; Published 5 May 2018) Abstract – The experimental research on the subject of human early embryo development has been remained insufficient because of ethical norms and legal constraints. In this context, the carrying out of theoretical studies could speed up the slow knowledge progression on this subject. Thus, I present here a particular model of theoretical study based on a synthesis of selected published experimental results combined with what has been provided from my interpretation of scientific signs masked in some Qur‟an verses. In the obtained detailed scenario, I consider that the third cleavage, resulting in 8 blastomeres, is coupled to a particular rearrangement in which 2 daughter cells seem move down and 2 others move up; while the 4 remaining cells seem stay at their initial position (I called them “4 HI cells”). Nevertheless, I consider that the fourth cleavage, resulting in 16 blastomeres, is coupled to a rearrangement that gives the impression of a harmonious descent: in fact, one of each two daughter cells seems pushed down. This descent materializes a morula top–bottom axis, which will go to coincide with the embryo-abembryo axis of the blastocyst. -

Spotsylvania County-Family Life Education
Spotsylvania County-Family Life Education Dear Parents and Guardians: During the year, Health & Physical Education teachers will teach a unit on Family Life Education. Please read the following objectives and “Opt out” procedure. Additionally, please review the state SOLs on the back of this form that may be covered during this unit. If you have any questions regarding content, procedures and/or opt out assignments, please contact your child’s teacher. Objective 4th Grade- physical changes during puberty, anatomy for reproduction and elimination, awareness of human fertilization and development, factors surrounding child abuse and neglect 5th Grade- connecting puberty to human anatomy and reproduction, mass media related to sexuality, abstinence, importance of nutrition and hygiene, recognition of threatening or uncomfortable situations, awareness of the existence of sexually transmitted infections “Opt Out” Procedure The General Assembly has mandated that all school divisions develop a Family Life Education Program. The regulations governing these programs, which were adopted by the Virginia State Board of Education, require that, an “Opt Out” procedure be established in which parents may elect for their children to be excused from all or part of the program. In Spotsylvania County Schools, the Parent/Community Involvement Team identified objectives for the program. Parents may “opt their children out” of any activities associated with these objectives. Copies of objectives for each grade level will be available at each school, and parents are invited to obtain copies of these objectives. Every effort has been made to select instructional materials and activities that reflect traditional family values our community can support. However, if parents wish their children to be excused from all or part of the program, they may complete the attached form (below) and return it to their teacher. -

26 Reproduction and Development
Reproduction 26 and Development Sex Determination Sex Chromosomes Determine Genetic Sex Sexual Diff erentiation Occurs Early in Development Basic Patterns of Reproduction Gametogenesis Begins in Utero The Brain Directs Reproduction Environmental Factors Infl uence Reproduction Male Reproduction Testes Produce Sperm and Hormones Spermatogenesis Requires Gonadotropins and Testosterone Male Accessory Glands Contribute Secretions to Semen Androgens Infl uence Secondary Sex Characteristics Female Reproduction Females Have an Internal Uterus The Ovary Produces Eggs and Hormones A Menstrual Cycle Lasts about One Month Hormonal Control of the Menstrual Cycle Is Complex Hormones Infl uence Female Secondary Sex Characteristics Procreation The Human Sexual Response Has Four Phases The Male Sex Act Includes Erection and Ejaculation Birth, and copulation, Sexual Dysfunction Aff ects Males and Females and death. That’s all Contraceptives Are Designed to Prevent Pregnancy the facts when you Infertility Is the Inability to Conceive come to brass tacks. Pregnancy and Parturition —T.S. Eliot, Sweeney Agonistes Fertilization Requires Capacitation The Developing Embryo Implants in the Endometrium The Placenta Secretes Hormones During Pregnancy Background Basics Pregnancy Ends with Labor and Delivery Positive and negative The Mammary Glands Secrete Milk During Lactation feedback Prolactin Has Other Physiological Roles Flagella Steroids Growth and Aging Agonist/antagonist Puberty Marks the Beginning of the Reproductive Years Up-and down-regulation Menopause and Andropause Are a Consequence of Aging Prostaglandins Hypothalamic-pituitary axis Prolactin Oxytocin Cross-section Spinal refl ex of intestinal villi Hot fl ashes (outlined in red). 900 Reproduction and Development magine growing up as a girl, then at the age of 12 or so, Sex hormones play a significant role in the behavior of fi nding that your voice is deepening and your genitals are other mammals, acting on adults as well as infl uencing the brain developing into those of a man. -
Cellular and Molecular Biology of Human Oogenesis, Ovulation And
干细胞之家www.stemcell8.cn ←点击进入 干细胞之家www.stemcell8.cn ←点击进入 干细胞之家www.stemcell8.cn ←点击进入 THIS PAGE IS BLANK 干细胞之家www.stemcell8.cn ←点击进入 干细胞之家www.stemcell8.cn ←点击进入 Copyright © 2008, NewAge International (P) Ltd., Publishers Published by New Age International (P) Ltd., Publishers All rights reserved. No part of this ebook may be reproduced in any form, by photostat, microfilm, xerography, or any other means, or incorporated into any information retrieval system, electronic or mechanical, without the written permission of the publisher. All inquiries should be emailed to [email protected] ISBN (10) : 81-224-2249-7 ISBN (13) : 978-81-224-2249-8 Price : £ 9.99 PUBLISHING FOR ONE WORLD NEWAGE INTERNATIONAL(P) LIMITED, PUBLISHERS 4835/24,Ansari Road, Daryaganj, New Delhi - 110002 Visit us at www.newagepublishers.com 干细胞之家www.stemcell8.cn ←点击进入 PREFACE The basic knowledge of ovarian development and maturation with special reference to formation of primordial oocyte, oocyte growth maturation, ovulation, fertilization, early development and biomedical and clinical implication of aging changes in oocytes, which are discussed in this book has number of applied aspects in immediate of long-term health prospects. Our knowledge of cellular and molecular biology of these aspects in the humans are of fundamental and applied interest to a wide variety of academic, scientific, toxicological, biomedical, biotechnological, clinical, disciplines for ensuring the normal early development and differentiation of human embryoes. Therefore, these aspects are discussed with an inter- disciplinary approach to produce knowledge that will help us in the better understanding of developmental disorders of children which are caused by developmental, maturational aging, genetic and environmental conditions such as chemical, xenobiotic factors, poor nutrition high ambient temperature, stress, improper management and inadequate health of developing child. -
Downloaded from Bioscientifica.Com at 09/24/2021 07:41:11PM Via Free Access 2 P Sutovsky
REPRODUCTIONREVIEW Sperm proteasome and fertilization Peter Sutovsky Division of Animal Sciences, and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri-Columbia, Columbia, Missouri 65211-5300, USA Correspondence should be addressed to P Sutovsky; Email: [email protected] Abstract The omnipresent ubiquitin–proteasome system (UPS) is an ATP-dependent enzymatic machinery that targets substrate proteins for degradation by the 26S proteasome by tagging them with an isopeptide chain composed of covalently linked molecules of ubiquitin, a small chaperone protein. The current knowledge of UPS involvement in the process of sperm penetration through vitelline coat (VC) during human and animal fertilization is reviewed in this study, with attention also being given to sperm capacitation and acrosome reaction/exocytosis. In ascidians, spermatozoa release ubiquitin-activating and conjugating enzymes, proteasomes, and unconjugated ubiquitin to first ubiquitinate and then degrade the sperm receptor on the VC; in echinoderms and mammals, the VC (zona pellucida/ZP in mammals) is ubiquitinated during oogenesis and the sperm receptor degraded during fertilization. Various proteasomal subunits and associated enzymes have been detected in spermatozoa and localized to sperm acrosome and other sperm structures. By using specific fluorometric substrates, proteasome-specific proteolytic and deubiquitinating activities can be measured in live, intact spermatozoa and in sperm protein extracts. The requirement of proteasomal proteolysis during fertilization has been documented by the application of various proteasome-specific inhibitors and antibodies. A similar effect was achieved by depletion of sperm-surface ATP. Degradation of VC/ZP-associated sperm receptor proteins by sperm-borne proteasomes has been demonstrated in ascidians and sea urchins. -

Robert G. Edwards for the Development of in Vitro Fertilization
PRESS RELEASE 2010-10-04 The Nobel Assembly at Karolinska Institutet has today decided to award The Nobel Prize in Physiology or Medicine 2010 to Robert G. Edwards for the development of in vitro fertilization SUMMARY Robert Edwards is aw arded the 2010 N obel Prize for the develop ment of human in vitro fertilization (IVF ) therapy. His achi evements have made it p ossible to tre at inf ertility, a medical condition afflicting a large proportion of humanity including more than 10% of all couples worldwide. As early as the 1950s, Edwards had the vision that IVF could be useful as a treatment f or infertility. He worked systematically to reali ze his goal, dis covered important p rinciples for human fertilization, and succeeded in accomplishing fertilization of human egg cells in test tubes ( or more precisely, cell culture d ishes).His efforts were f inally cro wned b y success on 25 July, 1 978, when the world´s first “test tube bab y” was born. During the following years, Edwa rds and his co-workers refined IVF technology and shared it with colleagues around the world. Approximately four million individ uals have so f ar been bornfollowing IVF. Man y of them are now adult and some have already become parents. A new field of medicinea sh emerged, with R obert Edwards leading t he process all the wa y from the f undamental discoveries to the curr ent, successful IVF therapy. His contributions represent a milestone in the development of modern medicine. The Nobel Assembly, consisting of 50 professors at Karolinska Institutet, awards the Nobel Prize in Physiology or Medicine. -

The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction
International Journal of Molecular Sciences Review The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction Emma R. James 1,2 , Douglas T. Carrell 1,2, Kenneth I. Aston 1, Timothy G. Jenkins 3, Marc Yeste 4 and Albert Salas-Huetos 1,* 1 Andrology and IVF Laboratory, Division of Urology, Department of Surgery, University of Utah School of Medicine, Salt Lake City, UT 84108, USA; [email protected] (E.R.J.); [email protected] (D.T.C.); [email protected] (K.I.A.) 2 Department of Human Genetics, University of Utah School of Medicine, Salt Lake City, UT 84112, USA 3 Department of Physiology and Developmental Biology, Brigham Young University, Provo, UT 84604, USA; [email protected] 4 Biotechnology of Animal and Human Reproduction (TechnoSperm), Unit of Cell Biology, Department of Biology, Faculty of Sciences, Institute of Food and Agricultural Technology, University of Girona, 17003 Girona, Spain; [email protected] * Correspondence: [email protected]; Tel.: +1-(385)-210-5534 Received: 7 July 2020; Accepted: 28 July 2020; Published: 29 July 2020 Abstract: It is well-established that testicular spermatozoa are immature and acquire motility and fertilization capabilities during transit throughout the epididymis. The epididymis is a duct-like organ that connects the testis to the vas deferens and is comprised of four anatomical regions: the initial segment, caput, corpus, and cauda. Sperm maturation occurs during epididymal transit by the interaction of sperm cells with the unique luminal environment of each epididymal region. In this review we discuss the epididymis as an essential reproductive organ responsible for sperm concentration, maturation (including sperm motility acquisition and fertilizing ability), protection and storage.