New Insights Into Human Early Embryo Development: a Particular Theoretical Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

When Does Human Life Begin? Christian Thinking and Contemporary Opposition
Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING The substance of this booklet is an extract from The Morning-After Pill – Uncovering the Truth, published by The Christian Institute in 2007: http://www.christian.org.uk/resource/the-morning-after-pill Copyright © The Christian Institute 2017 The author has asserted his right under Section 77 of the Copyright, Designs & Patents Act 1988 to be identified as the author of this work. First printed in June 2011 Reprinted in May 2015 and August 2017 ISBN 978-1-901086-47-8 Published by The Christian Institute Wilberforce House, 4 Park Road, Gosforth Business Park, Newcastle upon Tyne, NE12 8DG All rights reserved No part of this publication may be reproduced, or stored in a retrieval system, or transmitted, in any form or by any means, mechanical, electronic, photocopying, recording or otherwise, without the prior permission of The Christian Institute. All scripture quotations, unless otherwise indicated, are taken from the HOLY BIBLE, NEW INTERNATIONAL VERSION®. NIV®. Copyright © 1973, 1978, 1984 by International Bible Society. Used by permission of Zondervan. All rights reserved. The Christian Institute is a Company Limited by Guarantee, registered in England as a charity. Company No. 263 4440, Charity No. 100 4774. A charity registered in Scotland. Charity No. SC039220 Contents 5 1 . Introduction 7 2 . The answer from the Bible 17 3 . The view of the early church 21 4 . The drift from the biblical worldview 25 5 . -

Developmental Rate and Behavior of Early Life Stages of Bighead Carp and Silver Carp
Developmental Rate and Behavior of Early Life Stages of Bighead Carp and Silver Carp Scientific Investigations Report 2011–5076 U.S. Department of the Interior U.S. Geological Survey Cover images. Left to right (top) silver carp embryo at morula stage, silver carp embryo at olfactory placode stage, silver carp embryo at otolith appearance stage, (bottom) silver carp larvae at gill filament stage, silver carp larvae at one-chamber gas bladder stage. Developmental Rate and Behavior of Early Life Stages of Bighead Carp and Silver Carp By Duane C. Chapman and Amy E. George Scientific Investigations Report 2011–5076 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2011 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Chapman, D.C., George, A.E., 2011, Developmental rate and behavior of early life stages of bighead carp and silver carp: U.S. -

Cell Fate in the Early Mouse Embryo: Sorting out the Influence of Developmental History on Lineage Choice
Reproductive BioMedicine Online (2011) 22, 521– 524 www.sciencedirect.com www.rbmonline.com COMMENTARY Cell fate in the early mouse embryo: sorting out the influence of developmental history on lineage choice Samantha A Morris Wellcome Trust/Cancer Research UK Gurdon Institute, University of Cambridge, Tennis Court Road, Cambridge CB2 1QR, UK; University of Cambridge, Department of Physiology, Development and Neurobiology, Downing Street, Cambridge CB2 3DY, UK E-mail address: [email protected]. Abstract In early mouse embryos the first cell-fate decision segregates two cell populations: the outer trophectoderm (TE) and inner cell mass (ICM). Cells are primarily directed to the ICM in two waves of asymmetric division at the 8–16-cell and 16–32-cell stage transition – the first and second waves, respectively. The ICM then diverges to become epiblast (EPI) which will generate the embryo/fetus and extra-embryonic primitive endoderm (PE). Two recent studies have aimed to address the developmental origins of these lineages. Morris et al. (2010) found that first-wave-internalized cells mainly generate EPI, whereas later internalized cells pro- vide PE. This trend was not reflected in an independent study (Yamanaka et al., 2010). From direct comparison of both datasets, it becomes clear that the key difference lies in the proportions of cells internalized in the two waves, impacting greatly upon fate. When the majority of ICM is derived from only the first wave, both EPI and PE must differentiate from the available cells and no pattern is observed. Frequently though, closer parity exists between cells dividing asymmetrically in the first and second waves, revealing the influence of developmental history upon fate. -

Chapter 38: Reproduction and Development Computer Test Bank Cryopreservation
Chapter 38 Organizer Reproduction and Development Refer to pages 4T-5T of the Teacher Guide for an explanation of the National Science Education Standards correlations. Teacher Classroom Resources Activities/FeaturesObjectivesSection MastersSection TransparenciesReproducible Reinforcement and Study Guide, pp. 167-168 L2 Section Focus Transparency 93 L1 ELL Section 38.1 1. Identify the parts of the male and Inside Story: Sex Cell Production, p. 1033 Section 38.1 female reproductive systems. Problem-Solving Lab 38-1, p. 1035 Concept Mapping, p. 38 L3 ELL Basic Concepts Transparency 74 L2 ELL Human Reproductive 2. Summarize the negative feedback Human Critical Thinking/Problem Solving, p. 38 L3 Basic Concepts Transparency 75 L2 ELL Systems control of reproductive hormones. Reproductive Content Mastery, pp. 185-186, 188 L1 Reteaching Skills Transparency 55 L1P ELL National Science Education 3. Sequence the stages of the menstrual Systems P P cycle. Standards UCP.1-3, UCP.5; P C.1, C.5; F.1 (2 sessions, 11/ P 2 Reinforcement and Study Guide, p. 169 L2 P Section Focus Transparency 94 L1 ELLP blocks) Section 38.2 P LS BioLab and MiniLab Worksheets, pp. 169-170 L2 Reteaching Skills Transparency 56a,P 56b L1 LS P LS Development Laboratory Manual, pp. 277-284 L2 ELL P Before Birth LS Section 38.2 4. Summarize the events during each MiniLab 38-1: Examining Sperm and Egg Content Mastery, pp. 185,LS 187-188 L1 LS P LS trimester of pregnancy. Attraction, p. 1038 LS Development Before MiniLab 38-2: Making a Graph of Fetal Size, P LS P Reinforcement and Study Guide, p. -

Unit6:Human Reproduction Pregnancy and Embryonic Development Parturition and Lactation
H.S SECOND YEAR Unit6:Human Reproduction Pregnancy and embryonic development Parturition and lactation BY: Dr. LUNA PHUKAN HUMAN FERTILIZATION AND DEVELOPMENT Key Terms Term Meaning Gamete : A reproductive (sex) cell. In males, sperm; in females, eggs Fertilization : The process in sexual reproduction in which a male gamete and female gamete fuse to form a new cell Zygote : Cell resulting from fertilization Diploid (2n) : Cell that contains two sets of homologous chromosomes Haploid (n) : Cell that contains only a single set of genes Apoptosis :The process of programmed cell death Differentiation : The process by which cells become specialized in structure and function Human fertilization and development Fertilization is the process in which haploid gametes fuse to form a diploid cell called a zygote. To ensure that each zygote has the correct number of chromosomes, only one sperm can fuse with one egg. Stages of human development Zygotic stage: The zygote is formed when the male gamete (sperm) and female gamete (egg) fuse. Blastocyst stage: The single-celled zygote begins to divide into a solid ball of cells. Then, it becomes a hollow ball of cells called a blastocyst, attaching to the lining of the mother's uterus. Embryonic stage: The major internal organs and external features begin to emerge, forming an embryo. In this stage, the heart, brain, and spinal cord become visible. Arms and legs start to develop. Fetal stage: Once the formed features of the embryo begin to grow and develop, the organism is considered a fetus. Differentiation and specialization of structures happens during this time. REPRODUCTIVE SYSTEM REVIEW Key terms Term Meaning Gamete: A reproductive (sex) cell. -

From Cell Death to Embryo Arrest: Mathematical Models of Human Preimplantation Embryo Development
From cell death to embryo arrest: Mathematical models of human preimplantation embryo development K. Hardy*†‡, S. Spanos*, D. Becker†§, P. Iannelli†¶, R. M. L. Winston*, and J. Stark†¶ *Department of Reproductive Science and Medicine, Imperial College School of Medicine, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; §Department of Anatomy and Developmental Biology, ¶Centre for Nonlinear Dynamics and Its Applications, and †CoMPLEX (Centre for Mathematics and Physics in the Life Sciences and Experimental Biology), University College London, Gower Street, London WC1E 6BT, United Kingdom Communicated by Paul Nurse, Imperial Cancer Research Fund, London, United Kingdom, October 18, 2000 (received for review March 23, 2000) Human preimplantation embryos exhibit high levels of apoptotic generations during which individual cells can divide or die. The cells and high rates of developmental arrest during the first week relationship between the rate of cell death for individual cells in vitro. The relation between the two is unclear and difficult to and the data in Fig. 2 is therefore complex, precluding the direct determine by conventional experimental approaches, partly be- use of standard statistical tests. Instead, we construct a mathe- cause of limited numbers of embryos. We apply a mixture of matical model of the cell division and cell death process that experiment and mathematical modeling to show that observed allows us to relate parameters such as individual cell death rates levels of cell death can be reconciled with the high levels of embryo to global outcomes, such as the distribution of live and dead cell arrest seen in the human only if the developmental competence of numbers or the arrest or survival of the whole embryo. -

Tbn, a Novel Gene Essential for the ICM 5451
Development 127, 5449-5461 (2000) 5449 Printed in Great Britain © The Company of Biologists Limited 2000 DEV2556 Taube nuss is a novel gene essential for the survival of pluripotent cells of early mouse embryos Anne K. Voss*,‡,§, Tim Thomas*,‡, Petros Petrou, Konstantinos Anastassiadis1, Hans Schöler2 and Peter Gruss Max-Planck-Institute of Biophysical Chemistry, Department of Molecular Cell Biology, Am Fassberg 11, 37077 Goettingen, Germany 1European Molecular Biology Laboratory, Gene Expression, Meyerhofstr. 1, 69117 Heidelberg, Germany 2University of Pennsylvania, New Bolton Center, Center for Animal Transgenesis and Germ Cell Research, 382 W. Street Rd, Kennett Square, PA 19348, USA *These contributed equally to this work ‡Present address: Development and Neurobiology, The Walter and Eliza Hall Institute of Medical Research, Royal Parade, Parkville, Victoria 3050, Australia §Author for correspondence (e-mail: [email protected]) Accepted 19 September; published on WWW 14 November 2000 SUMMARY The cells of the inner cell mass constitute the pluripotent the zonae pellucidae, implanted and induced decidual cell population of the early embryo. They have the potential reactions, but failed to develop beyond E4.0. At this time to form all of the tissues of the embryo proper and the trophoblast cells were viable, but inner cell masses were some extra-embryonic tissues. They can be considered a not detectable. At E3.75, massive TUNEL-positive DNA transient stem cell population for the whole of the embryo, degradation and chromatin condensation were visible and stem cells maintaining the same capacity can be within the inner cell masses, whereas the cell membranes isolated from these cells. We have isolated, characterised where intact. -

Development Development
DEVELOPMENT DEVELOPMENT • Prenatal –Before birth • Postnatal development- After birth • PRENATAL DEVELOPMENT – 1. Embryonic development – Up to 8 weeks after fertilization. Devided into 23 arbitrarory stages called as Carnegie Stages Pre implantation development Post implantation development 2.Foetal development 8 weeks onwards after fertilization Cleavage ( post fertilization) • process of subdivision of ovum into smaller cells called cleavage. • process of repeated mitotic divisions of zygote occur with in zonapellucida, • these cells are known as blastomeres, • first cleavage division occur around 24 hrs after fertilization, • during 8 cell stage compaction of cells occur within the cells flatten & increase their intercellular contact , • Cleavage proceed to 16 celled stage --- MORULA, • All cells of approximately same size, • At 16 cells stage cells polarity is determuned to form outer trophoectoderm & inner cell mass, • inner cell mass give rise to embryo in future, while outer cell mass is destined to form the fetal membranes including placenta • the inner cell mass also called embryoblast , • cells of trophoblast help to provide nutrition to embryo, blastocyst • some fluid now passes into morula from uterine cavity , & partially separate the cells of inner cell mass from trophoblast. • as quantity of fluid increases the morula acquires shape of a cyst,the cells of trophoblast flattens out & inner cell mass gets attach to one side only, • the morula now is called blastocyst ,cavity is called blastocoele. • site where blastocyst is attach to inner cell mass is calld embryonic or animal pole , while opposite site is aembryonic pole. Zona pellucida( function) • trophoblast has property of being able to stick to uterine ( or other) epithelium & its cells have capacity to eat up other cells( property of invading) • thus as embryo is travelling down the uterine tube & uppermost part of uterine cavity , it is prevented from sticking to epithelium by a zona pellucida. -
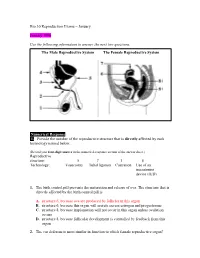
Numerical Response 1. Provide the Number of the Reproductive Structure That Is Directly Affected by Each Technology Named Below
Bio 30 Reproduction Exams – January January 1996 Use the following information to answer the next two questions. The Male Reproductive System The Female Reproductive System Numerical Response 1. Provide the number of the reproductive structure that is directly affected by each technology named below. (Record your four-digit answer in the numerical-response section of the answer sheet.) Reproductive structure: ______5__ ____7____ __1____ ___8_____ Technology: Vasectomy Tubal ligation Castration Use of an intrauterine device (IUD) 1. The birth control pill prevents the maturation and release of ova. The structure that is directly affected by the birth control pill is A. structure 6, because ova are produced by follicles in this organ B. structure 6, because this organ will secrete excess estrogen and progesterone C. structure 8, because implantation will not occur in this organ unless ovulation occurs D. structure 8, because follicular development is controlled by feedback from this organ 2. The vas deferens is most similar in function to which female reproductive organ? A. Ovary B. Uterus C. Vagina D. Fallopian tube Use the following information to answer the next question. Possible Effects of Testosterone 1 Inhibits skeletal muscle development 2 Enhances skeletal muscle development 3 Inhibits development of body hair 4 Promotes development of body hair 5 Inhibits gametogenesis 6 Stimulates gametogenesis 7 Enhances growth of the larynx 8 Inhibits growth of the larynx Numerical Response 2. Select all the correct effects of normal levels of testosterone in an adolescent male. (Record your answer in lowest-to-highest numerical order in the numerical-response section of the answer sheet.) Answer: __2467_____ Use the following information to answer the next two questions. -

Early Embryonic Development Till Gastrulation (Humans)
Gargi College Subject: Comparative Anatomy and Developmental Biology Class: Life Sciences 2 SEM Teacher: Dr Swati Bajaj Date: 17/3/2020 Time: 2:00 pm to 3:00 pm EARLY EMBRYONIC DEVELOPMENT TILL GASTRULATION (HUMANS) CLEAVAGE: Cleavage in mammalian eggs are among the slowest in the animal kingdom, taking place some 12-24 hours apart. The first cleavage occurs along the journey of the embryo from oviduct toward the uterus. Several features distinguish mammalian cleavage: 1. Rotational cleavage: the first cleavage is normal meridional division; however, in the second cleavage, one of the two blastomeres divides meridionally and the other divides equatorially. 2. Mammalian blastomeres do not all divide at the same time. Thus the embryo frequently contains odd numbers of cells. 3. The mammalian genome is activated during early cleavage and zygotically transcribed proteins are necessary for cleavage and development. (In humans, the zygotic genes are activated around 8 cell stage) 4. Compaction: Until the eight-cell stage, they form a loosely arranged clump. Following the third cleavage, cell adhesion proteins such as E-cadherin become expressed, and the blastomeres huddle together and form a compact ball of cells. Blatocyst: The descendents of the large group of external cells of Morula become trophoblast (trophoblast produce no embryonic structure but rather form tissues of chorion, extraembryonic membrane and portion of placenta) whereas the small group internal cells give rise to Inner Cell mass (ICM), (which will give rise to embryo proper). During the process of cavitation, the trophoblast cells secrete fluid into the Morula to create blastocoel. As the blastocoel expands, the inner cell mass become positioned on one side of the ring of trophoblast cells, resulting in the distinctive mammalian blastocyst. -

Formation of Germ Layers (Second & Third Week of Development)
8.12.2014 Formation of Germ Layers (Second & Third week of Development) Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow Day 8 • Blastocyst is partially embedded in the endometrial stroma. • Trophoblast differentiates into 2 layers: (i) Cytotrophoblast (ii) Syncytiotrophoblast • Cytotrophoblast shows mitotic division. Day 8 • Cells of inner cell mass (embryoblast) also differentiate into 2 layers: (i) Hypoblast layer (ii) Epiblast layer • Formation of amniotic cavity and embryonic disc. Day 9 • The blastocyst is more deeply embedded in the endometrium. • The penetration defect in the surface epithelium is closed by a fibrin coagulum. Day 9 • Large no. of vacuoles appear in syncytiotrophoblast which fuse to form lacunae which contains embryotroph. Day 9 • Hypoblast forms the roof of the exocoelomic cavity (primary yolk sac). • Heuser’s (exocoelomic membrane) • Extraembryonic mesoderm Day 11 & 12 • Formation of lacunar networks • Extraembryonic coelom (chorionic cavity) • Extraembryonic somatic mesoderm • Extraembryonic splanchnic mesoderm • Chorion Day 13 • Implantation bleeding • Villous structure of trophoblast. • Formation of Primary villi • Secondary (definitive) yolk sac • Chorionic plate (extraembronic mesoderm with cytotrophoblast) Third week of Development • Gastrulation (formation of all 3 germ layers) • Formation of primitive streak • Formation of notochord • Differentiation of 3 germ layers from Bilaminar to Trilaminar germ disc Formation of Primitive Streak (PS) • First sign of gastrulation • On 15th day • Primitive node • Primitive pit • Formation of mesenchyme on 16th day • Formation of embryonic endoderm • Intraembryonic mesoderm • Ectoderm • Epiblast is the source of all 3 germ layers Fate of Primitive Streak • Continues to form mesodermal cells upto early part of 4th week • Normally, the PS degenerates & diminishes in size.