Robert G. Edwards for the Development of in Vitro Fertilization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why the Medical Research Council Refused Robert Edwards and Patrick Steptoe Support for Research on Downloaded from Human Conception in 1971
Hum. Reprod. Advance Access published July 24, 2010 Human Reproduction, Vol.0, No.0 pp. 1–18, 2010 doi:10.1093/humrep/deq155 ORIGINAL ARTICLE Historical contribution Why the Medical Research Council refused Robert Edwards and Patrick Steptoe support for research on Downloaded from human conception in 1971 1,* 2 2 Martin H. Johnson , Sarah B. Franklin , Matthew Cottingham , http://humrep.oxfordjournals.org and Nick Hopwood 3 1Anatomy School and Trophoblast Research Centre, Department of Physiology, Development and Neuroscience, Anatomy School, University of Cambridge, Downing Street, Cambridge CB2 3DY, UK 2Department of Sociology & BIOS Centre, The London School of Economics and Political Science, Houghton Street, London WC2A 2AE, UK 3Department of History and Philosophy of Science, University of Cambridge, Free School Lane, Cambridge CB2 3RH, UK *Correspondence address. E-mail: [email protected] Submitted on March 30, 2010; accepted on May 26, 2010 background: In 1971, Cambridge physiologist Robert Edwards and Oldham gynaecologist Patrick Steptoe applied to the UK Medical at British Library of Political and Economic Science on July 27, 2010 Research Council (MRC) for long-term support for a programme of scientific and clinical ‘Studies on Human Reproduction’. The MRC, then the major British funder of medical research, declined support on ethical grounds and maintained this policy throughout the 1970s. The work continued with private money, leading to the birth of Louise Brown in 1978 and transforming research in obstetrics, gynaecology and human embryology. methods: The MRC decision has been criticized, but the processes by which it was reached have yet to be explored. Here, we present an archive-based analysis of the MRC decision. -

When Does Human Life Begin? Christian Thinking and Contemporary Opposition
Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING Salt&Light series When does human life begin? Christian thinking and contemporary opposition JOHN R LING The substance of this booklet is an extract from The Morning-After Pill – Uncovering the Truth, published by The Christian Institute in 2007: http://www.christian.org.uk/resource/the-morning-after-pill Copyright © The Christian Institute 2017 The author has asserted his right under Section 77 of the Copyright, Designs & Patents Act 1988 to be identified as the author of this work. First printed in June 2011 Reprinted in May 2015 and August 2017 ISBN 978-1-901086-47-8 Published by The Christian Institute Wilberforce House, 4 Park Road, Gosforth Business Park, Newcastle upon Tyne, NE12 8DG All rights reserved No part of this publication may be reproduced, or stored in a retrieval system, or transmitted, in any form or by any means, mechanical, electronic, photocopying, recording or otherwise, without the prior permission of The Christian Institute. All scripture quotations, unless otherwise indicated, are taken from the HOLY BIBLE, NEW INTERNATIONAL VERSION®. NIV®. Copyright © 1973, 1978, 1984 by International Bible Society. Used by permission of Zondervan. All rights reserved. The Christian Institute is a Company Limited by Guarantee, registered in England as a charity. Company No. 263 4440, Charity No. 100 4774. A charity registered in Scotland. Charity No. SC039220 Contents 5 1 . Introduction 7 2 . The answer from the Bible 17 3 . The view of the early church 21 4 . The drift from the biblical worldview 25 5 . -

Why the Medical Research Council Refused Robert Edwards and Patrick Steptoe Support for Research on Human Conception in 1971
Hum. Reprod. Advance Access published July 24, 2010 Human Reproduction, Vol.0, No.0 pp. 1–18, 2010 doi:10.1093/humrep/deq155 ORIGINAL ARTICLE Historical contribution Why the Medical Research Council refused Robert Edwards and Patrick Steptoe support for research on human conception in 1971 Martin H. Johnson 1,*, Sarah B. Franklin 2, Matthew Cottingham 2, and Nick Hopwood 3 Downloaded from 1Anatomy School and Trophoblast Research Centre, Department of Physiology, Development and Neuroscience, Anatomy School, University of Cambridge, Downing Street, Cambridge CB2 3DY, UK 2Department of Sociology & BIOS Centre, The London School of Economics and Political Science, Houghton Street, London WC2A 2AE, UK 3Department of History and Philosophy of Science, University of Cambridge, Free School Lane, Cambridge CB2 3RH, UK *Correspondence address. E-mail: [email protected] Submitted on March 30, 2010; accepted on May 26, 2010 http://humrep.oxfordjournals.org background: In 1971, Cambridge physiologist Robert Edwards and Oldham gynaecologist Patrick Steptoe applied to the UK Medical Research Council (MRC) for long-term support for a programme of scientific and clinical ‘Studies on Human Reproduction’. The MRC, then the major British funder of medical research, declined support on ethical grounds and maintained this policy throughout the 1970s. The work continued with private money, leading to the birth of Louise Brown in 1978 and transforming research in obstetrics, gynaecology and human embryology. methods: The MRC decision has been criticized, but the processes by which it was reached have yet to be explored. Here, we present an archive-based analysis of the MRC decision. results: We find evidence of initial support for Edwards and Steptoe, including from within the MRC, which invited the applicants to join its new directly funded Clinical Research Centre at Northwick Park Hospital. -

Chapter 38: Reproduction and Development Computer Test Bank Cryopreservation
Chapter 38 Organizer Reproduction and Development Refer to pages 4T-5T of the Teacher Guide for an explanation of the National Science Education Standards correlations. Teacher Classroom Resources Activities/FeaturesObjectivesSection MastersSection TransparenciesReproducible Reinforcement and Study Guide, pp. 167-168 L2 Section Focus Transparency 93 L1 ELL Section 38.1 1. Identify the parts of the male and Inside Story: Sex Cell Production, p. 1033 Section 38.1 female reproductive systems. Problem-Solving Lab 38-1, p. 1035 Concept Mapping, p. 38 L3 ELL Basic Concepts Transparency 74 L2 ELL Human Reproductive 2. Summarize the negative feedback Human Critical Thinking/Problem Solving, p. 38 L3 Basic Concepts Transparency 75 L2 ELL Systems control of reproductive hormones. Reproductive Content Mastery, pp. 185-186, 188 L1 Reteaching Skills Transparency 55 L1P ELL National Science Education 3. Sequence the stages of the menstrual Systems P P cycle. Standards UCP.1-3, UCP.5; P C.1, C.5; F.1 (2 sessions, 11/ P 2 Reinforcement and Study Guide, p. 169 L2 P Section Focus Transparency 94 L1 ELLP blocks) Section 38.2 P LS BioLab and MiniLab Worksheets, pp. 169-170 L2 Reteaching Skills Transparency 56a,P 56b L1 LS P LS Development Laboratory Manual, pp. 277-284 L2 ELL P Before Birth LS Section 38.2 4. Summarize the events during each MiniLab 38-1: Examining Sperm and Egg Content Mastery, pp. 185,LS 187-188 L1 LS P LS trimester of pregnancy. Attraction, p. 1038 LS Development Before MiniLab 38-2: Making a Graph of Fetal Size, P LS P Reinforcement and Study Guide, p. -

Unit6:Human Reproduction Pregnancy and Embryonic Development Parturition and Lactation
H.S SECOND YEAR Unit6:Human Reproduction Pregnancy and embryonic development Parturition and lactation BY: Dr. LUNA PHUKAN HUMAN FERTILIZATION AND DEVELOPMENT Key Terms Term Meaning Gamete : A reproductive (sex) cell. In males, sperm; in females, eggs Fertilization : The process in sexual reproduction in which a male gamete and female gamete fuse to form a new cell Zygote : Cell resulting from fertilization Diploid (2n) : Cell that contains two sets of homologous chromosomes Haploid (n) : Cell that contains only a single set of genes Apoptosis :The process of programmed cell death Differentiation : The process by which cells become specialized in structure and function Human fertilization and development Fertilization is the process in which haploid gametes fuse to form a diploid cell called a zygote. To ensure that each zygote has the correct number of chromosomes, only one sperm can fuse with one egg. Stages of human development Zygotic stage: The zygote is formed when the male gamete (sperm) and female gamete (egg) fuse. Blastocyst stage: The single-celled zygote begins to divide into a solid ball of cells. Then, it becomes a hollow ball of cells called a blastocyst, attaching to the lining of the mother's uterus. Embryonic stage: The major internal organs and external features begin to emerge, forming an embryo. In this stage, the heart, brain, and spinal cord become visible. Arms and legs start to develop. Fetal stage: Once the formed features of the embryo begin to grow and develop, the organism is considered a fetus. Differentiation and specialization of structures happens during this time. REPRODUCTIVE SYSTEM REVIEW Key terms Term Meaning Gamete: A reproductive (sex) cell. -

Founding Pioneers of IVF Update
Contents lists available at ScienceDirect Reproductive Biology journal homepage: www.elsevier.com/locate/repbio Review Founding Pioneers of IVF: Independent innovative researchers generating livebirths within 4 years of the first birth ⁎ John Lui Yovicha,b, , Ian Logan Craftc a PIVET Medical Centre, Perth, Western Australia, 6007, Australia b Curtin University, Perth, Western Australia, 6845, Australia c University of London, WC1E 7HU, England, United Kingdom ARTICLE INFO ABSTRACT Keywords: In this 40th anniversary year of the first IVF live birth, it is pertinent to look at all those teams endeavouring to In vitro fertilization, IVF generate live births from this unique technology and who succeeded within 4 years of the first. There were 9 Assisted reproductive technology, ART teams who achieved this and a further 3 who were successful soon after, by the end of 1982. This historical IVF history review is compiled by 2 authors who were actively engaged in the field of IVF at the time of the first birth and IVF teams who have remained active in Reproductive Medicine throughout their professional lives. They bring intimate Fathers of IVF and relevant knowledge of those pioneer researchers from the early years who can be classified as the "Founding Founding pioneers Reproductive medicine Pioneers" of IVF. 1. Introduction methodologies, several of the teams in this presentation were not pre- sent at that meeting, but achieved IVF live births by July 1982, working The first “test-tube baby”, Louise Joy Brown, was born at Oldham from independent endeavours. This was noted with surprise by John General Hospital situated in Manchester, England on 25th July 1978 Leeton [3] who observed that several advanced facilities were not re- [1]. -
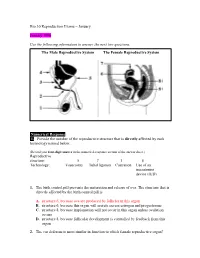
Numerical Response 1. Provide the Number of the Reproductive Structure That Is Directly Affected by Each Technology Named Below
Bio 30 Reproduction Exams – January January 1996 Use the following information to answer the next two questions. The Male Reproductive System The Female Reproductive System Numerical Response 1. Provide the number of the reproductive structure that is directly affected by each technology named below. (Record your four-digit answer in the numerical-response section of the answer sheet.) Reproductive structure: ______5__ ____7____ __1____ ___8_____ Technology: Vasectomy Tubal ligation Castration Use of an intrauterine device (IUD) 1. The birth control pill prevents the maturation and release of ova. The structure that is directly affected by the birth control pill is A. structure 6, because ova are produced by follicles in this organ B. structure 6, because this organ will secrete excess estrogen and progesterone C. structure 8, because implantation will not occur in this organ unless ovulation occurs D. structure 8, because follicular development is controlled by feedback from this organ 2. The vas deferens is most similar in function to which female reproductive organ? A. Ovary B. Uterus C. Vagina D. Fallopian tube Use the following information to answer the next question. Possible Effects of Testosterone 1 Inhibits skeletal muscle development 2 Enhances skeletal muscle development 3 Inhibits development of body hair 4 Promotes development of body hair 5 Inhibits gametogenesis 6 Stimulates gametogenesis 7 Enhances growth of the larynx 8 Inhibits growth of the larynx Numerical Response 2. Select all the correct effects of normal levels of testosterone in an adolescent male. (Record your answer in lowest-to-highest numerical order in the numerical-response section of the answer sheet.) Answer: __2467_____ Use the following information to answer the next two questions. -

Robert Edwards (1925–2013) Pioneer of in Vitro Fertilization
COMMENT OBITUARY Robert Edwards (1925–2013) Pioneer of in vitro fertilization. everal scientists have made discoveries In 1980, Edwards and Steptoe founded a that have saved millions of lives. Robert private fertility clinic at Bourn Hall, outside Edwards helped to create them. Cambridge. Edwards wanted to make IVF SEdwards, who died on 10 April, was born acceptable but also, as the father of five daugh- in 1925 in Batley, UK, a West Yorkshire mill ters, to speak up for people who were infer- town, and educated in Manchester. He stud- tile. Edwards was their champion in ethically ied agriculture and zoology at the University charged battles with scientists, theologians, of Wales, in Bangor, UK, after nearly four politicians and even Nobel laureates, whose STUDIO/SPL CORBIN O’GRADY years of military service. In 1951, he gradu- pantheon he later joined. His hopes were ated with only a pass. Despite this inauspi- vindicated, but success had come at the price cious start, his friend John Slee remembered of being accused of everything from killing much later that Edwards had been “ambi- embryonic ‘babies’ to courting the media. tious and flexible, and unusually confident Our beloved professor could be madden- in his own judgement”. ing when he rolled out ideas for experiments Soon after Edwards had enrolled at the like a newspaper press. The late grande dame University of Edinburgh, UK, to pursue a of embryology, Anne McLaren, a contempo- diploma in animal genetics, his professor, rary of Bob’s, once told me: “From scores of Conrad Waddington, offered him a PhD ideas, some gems he digs up sparkle so bril- studentship and later a fellowship. -

Embryology Text Books: Implications for Fetal Research Dianne N
The Linacre Quarterly Volume 61 | Number 2 Article 6 May 1994 "New Age" Embryology Text Books: Implications for Fetal Research Dianne N. Irving Follow this and additional works at: https://epublications.marquette.edu/lnq Recommended Citation Irving, Dianne N. (1994) ""New Age" Embryology Text Books: Implications for Fetal Research," The Linacre Quarterly: Vol. 61 : No. 2 , Article 6. Available at: https://epublications.marquette.edu/lnq/vol61/iss2/6 "New Age" Embryology Text Books: Implications for Fetal Research by Dianne N. Irving, M.A., Ph.D. The author is Assistant Professor, History ofPhilosophy/Bioethies at De Sales School of Theology, Washington, D. C. As outrageous as it is that so much incorrect science has been and still is being used in the scientific, medical and bioethics literature to argue that fetal "personhood" does not arrive until some magical biological marker event during human embryological development, now we can witness the "new wave" consequences of passively allowing such incorrect "new age" science to be published and eventually accepted by professionals and non-professionals alike. Once these scientifically erroneous claims, and the erroneoliS philosophical and theological concepts they engender, are successfully imbedded in these bodies of literature and in our collective consciousnesses, the next logical step is to imbed them in our text books, reference materials and federal regulations. Such is the case with the latest fifth edition of a highly respected embryology text book by Keith Moore - The Developing Human. 1 This text is used in most medical schools and graduate biology departments here, and in many institutions abroad. Several definitions and redefinitions of scientific terms it uses are incorporated, it would seem, in order to support the "new age" political agenda of abortion and fetal research. -

New Insights Into Human Early Embryo Development: a Particular Theoretical Study
14 International Journal of Modern Anthropology Int. J. Mod. Anthrop. (2018) Vol: 2, Issue No: 11, pp: 14 - 46 Available online at: www.ata.org.tn ; DOI: http://dx.doi.org/10.4314/ijma.v2i11.1 Original Synthesis Article New insights into human early embryo development: a particular theoretical study Hassen Chaabani Laboratory of Human Genetics and Anthropology, Faculty of Pharmacy, University of Monastir, 5000 Monastir, Tunisia. E.mail: [email protected] (Received 18 January 2018; Accepted 5 Mars 2018; Published 5 May 2018) Abstract – The experimental research on the subject of human early embryo development has been remained insufficient because of ethical norms and legal constraints. In this context, the carrying out of theoretical studies could speed up the slow knowledge progression on this subject. Thus, I present here a particular model of theoretical study based on a synthesis of selected published experimental results combined with what has been provided from my interpretation of scientific signs masked in some Qur‟an verses. In the obtained detailed scenario, I consider that the third cleavage, resulting in 8 blastomeres, is coupled to a particular rearrangement in which 2 daughter cells seem move down and 2 others move up; while the 4 remaining cells seem stay at their initial position (I called them “4 HI cells”). Nevertheless, I consider that the fourth cleavage, resulting in 16 blastomeres, is coupled to a rearrangement that gives the impression of a harmonious descent: in fact, one of each two daughter cells seems pushed down. This descent materializes a morula top–bottom axis, which will go to coincide with the embryo-abembryo axis of the blastocyst. -

History of Clinical IVF
Knowing Where We Came From: History of Clinical IVF Thomas B. Pool, Ph.D., HCLD Fertility Center of San Antonio San Antonio, Texas Disclosures “The vast majority of human beings dislike and even actually dread all notions with which they are not familiar…Hence, it comes about that at their first appearance, innovators have generally been persecuted, and always, derided as fools and madmen.” Aldous Huxley author Brave New World, 1932 “Most human beings have an almost infinite capacity for taking things for granted”. Acknowledgements I want to thank the following individuals for their altruism and assistance in preparing this presentation for the College of Reproductive Biology: Peter Brinsden (l) with James Watson and Bob Edwards Lucinda Veeck Gosden Kay Elder Professor Sir Richard L. Gardner Jacques Cohen Cohen, Jacques, 2013. From Pythagoras and Aristotle to Boveri and Edwards: a history of clinical embryology and therapeutic IVF. In: Textbook of Clinical Embryology (Coward K., Wells, D.,eds) Cambridge University Press, p177-102. Henry Leese The early history of IVF 1 • Aristotle (384-322 BC) proposed the theory that children are a product of “the mingling of male and female seed”. This opposed the prevailing theory that children were from the male seed and women merely the “receptacle for the child”. • William Harvey (1578-1657) studied the fertility of the King’s herd of deer, and wrote: “De generatione animalium” in1651, in which occurs the well known phrase: “Ex ovo omnia” – “from the egg is everything”. • Antonj van Leeuwenhoek (1632-1723) carried out the first studies on human sperm with the newly invented microscope. -

ART and the Art of Medicine
Virtual Mentor American Medical Association Journal of Ethics January 2014, Volume 16, Number 1: 3-4. FROM THE EDITOR ART and the Art of Medicine On a summer night in 1978, as the hour approached midnight, Dr. Patrick Steptoe prepared himself for surgery [1]. A veteran obstetrician and gynecologist nearing retirement, Dr. Steptoe had delivered many babies by caesarean section, and he performed the surgery flawlessly [1]. At 11:47 pm, Louise Joy Brown came into the world, perfect and exquisite and vulnerable in that way only babies can be, though Louise Joy Brown was no ordinary baby [1]. To her parents, she was an unexpected and yearned-for gift after almost 10 years of trying to conceive; to Dr. Steptoe and his colleague, Robert Edwards, she was the culmination of countless years of research; and, to the world, she was a mixture of scientific triumph and medical marvel. She was the first so called “test tube baby,” or, in the correct scientific parlance, the first baby born following in vitro fertilization (IVF), which entails extracting eggs from a woman using a needle, fertilizing one or more with sperm, and then inserting one or more of the embryos into her uterus with the hopes that one implants. This year, a pregnant Louise celebrated her thirty-fifth birthday, with her husband and their first child [2]. In those 35 years since Louise’s birth, the field of assisted reproductive technology (ART) has flourished. What was on that July night a scientific breakthrough has become commonplace. Couples who are carriers for devastating genetic conditions they don’t want to pass on, gay and lesbian couples, women with anatomic abnormalities that prevent them from conceiving, men with abnormalities in their sperm, and countless others have found ART to be a kind of savior science, a way to have children when nature has denied them that option.