Federal Support for Reproductive Health Services: Frequently Asked Questions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sex-Selective Abortions, Fertility, and Birth Spacing*
Sex-Selective Abortions, Fertility, and Birth Spacing* Claus C Portner¨ Department of Economics Albers School of Business and Economics Seattle University, P.O. Box 222000 Seattle, WA 98122 [email protected] www.clausportner.com & Center for Studies in Demography and Ecology University of Washington November 2016 *I am grateful to Andrew Foster and Darryl Holman for discussions about the method. I owe thanks to Shelly Lundberg, Daniel Rees, David Ribar, Hendrik Wolff, seminar participants at University of Copenhagen, University of Michigan, University of Washington, University of Arhus,˚ the Fourth Annual Conference on Population, Repro- ductive Health, and Economic Development, and the Economic Demography Workshop for helpful suggestions and comments. I would also like to thank Nalina Varanasi for research assistance. Support from the University of Wash- ington Royalty Research Fund and the Development Research Group of the World Bank is gratefully acknowledged. The views and findings expressed here are those of the author and should not be attributed to the World Bank or any of its member countries. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, 5R24HD042828, to the Center for Studies in Demography and Ecology at the University of Washington. Prior versions of this paper were presented under the title “The Determinants of sex-selective Abortions.” Abstract This paper addresses two main questions: what is the relationship between fertility and sex se- lection and how does birth spacing interact with the use of sex-selective abortions? I introduce a statistical method that incorporates how sex-selective abortions affect both the likelihood of a son and spacing between births. -

World Fertility and Family Planning 2020: Highlights (ST/ESA/SER.A/440)
World Fertility and Family Planning 2020 Highlights ST/ESA/SER.A/440 Department of Economic and Social Affairs Population Division World Fertility and Family Planning 2020 Highlights United Nations New York, 2020 The Department of Economic and Social Affairs of the United Nations Secretariat is a vital interface between global policies in the economic, social and environmental spheres and national action. The Department works in three main interlinked areas: (i) it compiles, generates and analyses a wide range of economic, social and environmental data and information on which States Members of the United Nations draw to review common problems and take stock of policy options; (ii) it facilitates the negotiations of Member States in many intergovernmental bodies on joint courses of action to address ongoing or emerging global challenges; and (iii) it advises interested Governments on the ways and means of translating policy frameworks developed in United Nations conferences and summits into programmes at the country level and, through technical assistance, helps build national capacities. The Population Division of the Department of Economic and Social Affairs provides the international community with timely and accessible population data and analysis of population trends and development outcomes for all countries and areas of the world. To this end, the Division undertakes regular studies of population size and characteristics and of all three components of population change (fertility, mortality and migration). Founded in 1946, the Population Division provides substantive support on population and development issues to the United Nations General Assembly, the Economic and Social Council and the Commission on Population and Development. It also leads or participates in various interagency coordination mechanisms of the United Nations system. -

FBI Independence As a Threat to Civil Liberties: an Analogy to Civilian Control of the Military
\\jciprod01\productn\G\GWN\86-4\GWN403.txt unknown Seq: 1 30-AUG-18 9:12 FBI Independence as a Threat to Civil Liberties: An Analogy to Civilian Control of the Military Justin Walker* ABSTRACT At a time when the President is under investigation, and in the wake of a controversial dismissal of the FBI Director, the need for an “independent” FBI has appeared to many to be more important than ever. Indeed, the Senate would not have confirmed the new FBI Director, Christopher Wray, if he had not promised to be independent of the President and the Attorney General. This Article argues that calls for an independent FBI are misguided and dan- gerous. The Article analogizes presidential control of the FBI to civilian con- trol of the military by demonstrating that, contrary to conventional wisdom, the FBI and the military share the same purpose. It then explores in depth how the FBI has often infringed on civil liberties in the same way that the framers worried an out-of-control military might do so, and it explains why the inde- pendence that the FBI has often enjoyed was a cause of those violations. Fi- nally, it concludes that if it is necessary to preserve the FBI’s investigative independence, the solution is to split the FBI to reflect the model of many western democracies—creating an independent agency to investigate crime (like Britain’s New Scotland Yard) and a separate agency to continue the FBI’s national security functions (like Britain’s MI5). TABLE OF CONTENTS INTRODUCTION ................................................. 1012 R I. -

Family Planning and the Environment
FAMILY PLANNING AND THE ENVIRONMENT STABILIZING POPULATION WOULD HELP SUSTAIN THE PLANET Because everyone counts ABOUT HALF THE EARTH’s biological people’s needs and that face the greatest production capacity has already been di- population growth. verted to human use. Life-supporting eco- z Since the 1960s, fertility in de- systems are affected everywhere by the veloping countries has been reduced planet’s 6.7 billion people, which is pro- from an average of six births per jected to reach at least 9.2 billion by 2050. woman to three, thanks primarily to The links between population the use of contraceptives. However, UNFPA, the United Nations and environmental quality are com- in 56 developing countries, the poorest plex and varied. Understanding them women still average six births, compared Population Fund, is an requires knowledge of consumption rates to 3.2 for the wealthiest. that differ between the rich and the poor, international development z The wealthiest countries, with less than of new and old technologies, of resource 20 per cent of earth’s population and the agency that promotes the extraction and restoration, and of the dy- slowest population growth, account for 86 namics of population growth and migration. right of every woman, man percent of natural resource consumption– Humans are depleting natural re- much of it wasteful–and produce the ma- and child to enjoy a life of sources, degrading soil and water, and cre- jority of the pollution and carbon dioxide. ating waste at an alarming rate, even as health and equal opportunity. z At the other extreme, the depletion of new technology raises crop yields, con- natural resources is occurring most rapidly serves resources and cleans up pollution. -

Pre and Interconception Health, Intentionality and Birth Spacing: Emerging Issues
PRE AND INTERCONCEPTION HEALTH, INTENTIONALITY AND BIRTH SPACING: EMERGING ISSUES Monday, May 21 1:30 PM - 3:00 PM #prematuritycollab Pre- and Interconception Health, Intentionality, and Birth Spacing: Emerging Approaches Prematurity Prevention Summit Building a Birth Equity Movement Welcome • Co-chairing the Prematurity Collaborative Clinical and Public Health Practice Workgroup ‣ With Vanessa Lee, MPH (HRSA) • Great group of people; very dedicated • Focus on 3 main topics: ‣ 17-P ‣ LDASA ‣ Intentionality and birth spacing Background • Approximately half of pregnancies in US are unintended ‣ Better in teens, but still unacceptably high • Intentionality is important ‣ Unintended pregnancy associated with adverse maternal and child outcomes ‣ Negative impact on physical and mental health ‣ Disproportionate impact › Higher rates in lower SES and lower levels of achieved education ‣ Expensive… › 2010 estimate: $21 billion in public expenditure for unintended pregnancy Background – Birth Spacing • Based on data suggesting short interpregnancy intervals associated with worse outcomes ‣ Including prematurity ‣ Others: fetal growth restriction, NICU admission, perinatal death • Intervals “debated”…. ‣ < 24 months, especially < 18 months • “Recommendations” to avoid short intervals ‣ Healthy People 2020 objective: 10% reduction in pregnancies within 18 months ‣ WHO: minimum birth-to-pregnancy spacing of 2 years ‣ ACOG: short intervals mentioned as a risk factor for preterm delivery and adverse neonatal outcomes • Widespread emphasis ‣ Made sense -

Serving Those Who Serve?
Serving Those Who Serve? Restrictions on abortion Access to safe, legal abortion services is essential to access for servicemembers, a person’s health and central to their economic and social veterans, and their well-being. Everyone deserves access to, and comprehensive dependents coverage for, safe abortion services in their communities, and that includes members of our military and their families. Yet servicemembers and their dependents, as well as veterans, face unjustified, hardline restrictions on their access to abortion. Federal law prohibits the Department Overall, the rate of unintended pregnancy of Defense from providing abortion in the Armed Forces is higher than among services at military treatment facilities, the general population.5 An analysis of the and the TRICARE insurance program 2011 Survey of Health Related Behaviors from covering such services, except when found that seven percent of active-duty a pregnancy is the result of rape, incest or women of reproductive age reported an when the life of the pregnant person is at unintended pregnancy in the previous risk. The Veterans Health Administration year. That same year, 4.5% of women of (VHA), which provides health services to reproductive age in the general U.S. popu- veterans, does not provide or cover abor- lation reported an unintended pregnancy.6 tion services under any circumstances. This issue brief — the first in a three-part Bans on abortion care and coverage series — discusses the unique barriers adversely impact veterans, servicemem- servicemembers, veterans and their bers and their families, including the dependents face in accessing abortion women and transgender men whose care. First, we explain the restrictions on service is vital to the national security abortion for active duty servicemembers of the United States. -

Family Planning Services APPOINTMENTS COMPREHENSIVE CONTRACEPTIVE SERVICES We Take All Major Insurance Carriers
DUKE OBGYN Family Planning Services APPOINTMENTS COMPREHENSIVE CONTRACEPTIVE SERVICES We take all major insurance carriers. We can also offer financial assistance to uninsured or underinsured patients paying out of pocket. Please call our Family Planning Coordinator directly at 919-668- 7888 for more information or to make an appointment. LOCATION Duke South Clinic 1J 200 Trent Drive Durham, NC 27710 CONTACT Tel: 919-668-7888 Fax: 919-681-4838 OBGYN.DUKE.EDU/FAMILY PLANNING We are pleased to offer a focused family planning program at Duke University Medical Center. Our attending physicians are faculty of the Department of Obstetrics and Gynecology and are specialists in the field of family planning. We specialize in innovative contraceptive techniques and provide MEDICAL AND We provide medical and surgical termination comprehensive contraceptive services, especially for patients with SURGICAL of pregnancy in a private and specialized complex medical issues. TERMINATION environment. Our services include management OF PREGNANCY of complicated pregnancies, mid-trimester Some of these conditions include hypertension (high blood pressure), pregnancies, and terminations for fetal diabetes, coagulation disorders (blood clotting problems), history of anomalies. All patients receive counseling regarding pregnancy options and blood clots (deep vein thrombosis or pulmonary embolism), cardiac procedures, ultrasound confirmation of pregnancy dating, and contraceptive disease, migraines, extensive uterine or cervical surgeries, or a options. We provide 24-hour patient phone contact for emergencies. compromised immune system. CONTRACEPTIVE SERVICES WE OFFER INCLUDE: MANAGEMENT OF We provide services for women experiencing MISCARRIAGE miscarriage, including medical treatment and • Sterilization including Essure tubal occlusion procedure surgical management of pregnancy loss in a (www.essure.com) private, specialized and compassionate outpatient environment. -

Pregnancy Tests for Family Planning
PRODUCT BRIEF Caucus on New and Underused Reproductive Health Technologies Pregnancy tests for family planning Description • Early access to pregnancy tests was associated with earlier access to antenatal care or abortion services in a Low-cost, accurate urine pregnancy tests are a simple study in South Africa.11 tool that can be used to rule out pregnancy for some women and help increase access to same-day provision of family planning methods.* Guidance from the World Efficacy Health Organization (WHO) indicates that, for hormonal contraceptives, a woman can initiate a method if her Proper use and accuracy heath care provider is “reasonably certain she is not Two types of urine pregnancy tests are currently available. 1 pregnant.” Because many family planning providers Both types employ test strips that detect human chorionic in developing countries rely on the presence of menses gonadotropin (hCG) levels in the urine to determine the to rule out pregnancy among clients, women who are likelihood of pregnancy. In the first type, the user holds not menstruating at the time they visit the clinic are a test strip in the urine stream to capture a mid-stream 2,3 routinely denied same-day provision of family planning. sample. In the second type, the user captures a urine Studies show that anywhere from 5 to 50 percent of non- sample in a cup and then dips a test strip into the cup 4,5 menstruating women are denied services, even though (known as a “dip strip test”). With both types, the user several studies have shown that very few of these women only has to wait a few minutes before viewing the results 6,7 are actually pregnant. -

Uniformed Services Employment and Reemployment Rights Act (USERRA)
Uniformed Services Employment and Reemployment Rights Act (USERRA) - ------------------ ----------------------- District of Arizona 40 N. Central, Suite 1200 Phoenix, Arizona 85004 Table of Contents Arizona Facts and Figures…………………………………….1 Uniformed Services Employment Reemployment Rights Act (USERRA)……………………………………….….2 Making It Easier for Civilian Employers of Those Who Serve in the National Guard and Reserve ………………………………………………………10 USERRA FAQs for Employers…………………………….12 A Smooth Transition for National Guard and Reserve Members Avoiding Job Conflicts ………..16 USERRA FAQs for Service Members.………………..19 Employment Rights and Benefits of Federal Civilian Employees Who Perform Active Military Duty………………………………………………………23 Veterans’ Reemployment Rights (VRR)……………30 Family and Medical Leave Act (FMLA)………………34 USERRA – A Quick Look……………………………………36 USERRA Complaints………………………………………….41 USERRA - Veterans’ Rights………………………..…….43 Answers to Frequently Asked Questions About The 2302(c) Program ……………………………………….45 Resources…………………………………………………….…...47 Arizona Fact and Figures Major Installations Army • Fort Huachuca • Navajo Army Depot, Flagstaff • Papago Park, Phoenix • Barnes Reserve Center, Phoenix • Herrera Reserve Center, Mesa Navy & Marine Corps • Yuma Proving Grounds • Yuma Naval Air Station Air Force • Luke AFB • Davis Monthan AFB Disclaimer This pamphlet is intended to be a non-technical resource for informational purposes only. Its contents are not legally binding nor should it be considered as a substitute for the language of the actual statute or the official USERRA Handbook. USERRA The Uniformed Services Employment and Reemployment Rights Act (USERRA) was enacted to ensure that members of the uniformed services are entitled to return to their civilian employment upon completion of their service. They should be reinstated with the seniority, status, and rate of pay they would have obtained had they remained continuously employed by their civilian employer. -

Treatment of American Prisoners of War in Southeast Asia 1961-1973 by John N. Powers
Treatment of American Prisoners of War In Southeast Asia 1961-1973 By John N. Powers The years 1961 to 1973 are commonly used when studying American POWs during the Vietnam War, even though history books generally refer to the years 1964 to 1973 in defining that war. Americans were captured as early as 1954 and as late as 1975. In these pages the years 1961 to 1973 will be used. Americans were held prisoner by the North Vietnamese in North Vietnam, the Viet Cong (and their political arm the National Liberation Front) in South Vietnam, and the Pathet Lao in Laos. This article will not discuss those Americans held in Cambodia and China. The Defense Prisoner of War/Missing Personnel Office (DPMO) lists 687 American Prisoners of War who were returned alive by the Vietnamese from 1961 through 1976. Of this number, 72 were returned prior to the release of the bulk of the POWs in Operation Homecoming in 1973. Twelve of these early releases came from North Vietnam. DPMO figures list thirty-six successful escapes, thirty-four of them in South Vietnam and two in Laos. There were more than those thirty-six escapes, including some from prison camps in Hanoi itself. Some escapes ended in recapture within hours, some individuals were not recaptured for days, and some were simply never seen again. There were individuals who escaped multiple times, in both North and South Vietnam. However, only thirty- six American prisoners of war escaped and reached American forces. Of those thirty- six successful attempts, twenty-eight of them escaped within their first month of captivity. -

Securing Equal Justice for All
Sec Eql Justce_Cvr 2/7/07 8:45 AM Page 2 Securing Equal Justice for All: A Brief History of Civil Legal Assistance in the United States BY ALAN W. HOUSEMAN LINDA E. PERLE Revised January 2007 CENTER FOR LAW AND SOCIAL POLICY Sec Eql Justce 2/5/07 10:25 AM Page i Securing Equal Justice for All: A Brief History of Civil Legal Assistance in the United States BY ALAN W. HOUSEMAN LINDA E. PERLE Revised January 2007 CENTER FOR LAW AND SOCIAL POLICY Sec Eql Justce 2/5/07 10:25 AM Page ii Acknowledgements This short history is based on the previous written work of Justice Earl Johnson, Justice John Dooley, Martha Bergmark, and the authors. We want to thank all of those who reviewed the manuscript and made helpful comments, which significantly improved the accuracy and substance of the piece. These reviewers include: Jon Asher, Hulett (Bucky) Askew, Earl Johnson, Victor Geminiani, Bill McCalpin, and Don Saunders. We also want to thank Gayle Bennett for her efforts to pull together assorted materials the authors had previously written and for her editorial assistance. The Center for Law and Social Policy (CLASP) serves as counsel to the National Legal Aid and Defender Association (NLADA) and its member programs. Securing Equal Justice for All: A Brief History of Civil Legal Assistance About the Authors Alan W. Houseman is CLASP’s Executive Director. Mr. Houseman has written widely about- civil legal assistance to the poor and has been directly involved in many of the initiatives described in this paper. -
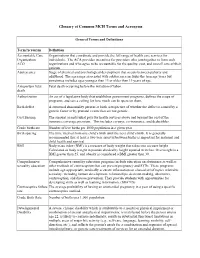
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height.