New Zealand Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
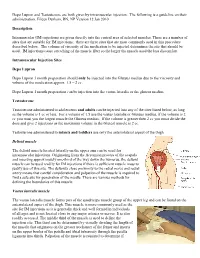
An Intramuscular Injection Is an Injection Given Directly Into The
Depo Lupron and Testosterone are both given by intramuscular injection. The following is a guideline on their administration. Eileen Durham, RN, NP Version 12 Jan 2010 Description Intramuscular (IM) injections are given directly into the central area of selected muscles. There are a number of sites that are suitable for IM injections; there are three sites that are most commonly used in this procedure described below. The volume of viscosity of the medication to be injected determines the site that should be used. IM injections cause stretching of the muscle fiber so the larger the muscle used the less discomfort. Intramuscular Injection Sites Depo Lupron Depo Lupron 3 month preparation should only be injected into the Gluteus medius due to the viscosity and volume of the medication approx. 1.5 – 2 cc. Depo Lupron 1 month preparation can be injection into the vastus lateralis or the gluteus medius. Testosterone Testosterone administered to adolescents and adults can be injected into any of the sites listed below, as long as the volume is 1 cc or less. For a volume of 1.5 use the vastus lateralis or Gluteus medius, if the volume is 2 cc you must you the largest muscle the Gluteus medius. If the volume is greater then 2 cc you must divide the dose and give 2 injections as the maximum volume in the Gluteal muscle is 2 cc. Testosterone administered to infants and toddlers use only the anteriolateral aspect of the thigh. Deltoid muscle The deltoid muscle located laterally on the upper arm can be used for intramuscular injections. -

FSI-D-16-00226R1 Title
Elsevier Editorial System(tm) for Forensic Science International Manuscript Draft Manuscript Number: FSI-D-16-00226R1 Title: An overview of Emerging and New Psychoactive Substances in the United Kingdom Article Type: Review Article Keywords: New Psychoactive Substances Psychostimulants Lefetamine Hallucinogens LSD Derivatives Benzodiazepines Corresponding Author: Prof. Simon Gibbons, Corresponding Author's Institution: UCL School of Pharmacy First Author: Simon Gibbons Order of Authors: Simon Gibbons; Shruti Beharry Abstract: The purpose of this review is to identify emerging or new psychoactive substances (NPS) by undertaking an online survey of the UK NPS market and to gather any data from online drug fora and published literature. Drugs from four main classes of NPS were identified: psychostimulants, dissociative anaesthetics, hallucinogens (phenylalkylamine-based and lysergamide-based materials) and finally benzodiazepines. For inclusion in the review the 'user reviews' on drugs fora were selected based on whether or not the particular NPS of interest was used alone or in combination. NPS that were use alone were considered. Each of the classes contained drugs that are modelled on existing illegal materials and are now covered by the UK New Psychoactive Substances Bill in 2016. Suggested Reviewers: Title Page (with authors and addresses) An overview of Emerging and New Psychoactive Substances in the United Kingdom Shruti Beharry and Simon Gibbons1 Research Department of Pharmaceutical and Biological Chemistry UCL School of Pharmacy -

Octopamine and Tyramine Regulate the Activity of Reproductive Visceral Muscles in the Adult Female Blood-Feeding Bug, Rhodnius Prolixus Sam Hana* and Angela B
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 1830-1836 doi:10.1242/jeb.156307 RESEARCH ARTICLE Octopamine and tyramine regulate the activity of reproductive visceral muscles in the adult female blood-feeding bug, Rhodnius prolixus Sam Hana* and Angela B. Lange ABSTRACT Monastirioti et al., 1996). Octopamine and tyramine signal via The role of octopamine and tyramine in regulating spontaneous G-protein coupled receptors (GPCRs), leading to changes in second contractions of reproductive tissues was examined in the messenger levels. The recently updated receptor classification α β female Rhodnius prolixus. Octopamine decreased the amplitude of (Farooqui, 2012) divides the receptors into Oct -R, Oct -Rs β β β spontaneous contractions of the oviducts and reduced RhoprFIRFa- (Oct 1-R, Oct 2-R, Oct 3-R), TYR1-R and TYR2-R. In general, β α induced contractions in a dose-dependent manner, whereas tyramine Oct -Rs lead to elevation of cAMP while Oct -R and TYR-Rs lead to 2+ only reduced the RhoprFIRFa-induced contractions. Both octopamine an increase in Ca (Farooqui, 2012). and tyramine decreased the frequency of spontaneous bursal The movement of eggs in the reproductive system of contractions and completely abolished the contractions at Rhodnius prolixus starts at the ovaries, the site of egg maturation. 5×10−7 mol l−1 and above. Phentolamine, an octopamine receptor Upon ovulation, mature eggs are released into the oviducts antagonist, attenuated the inhibition induced by octopamine on the (Wigglesworth, 1942). Eggs are then guided, via oviductal oviducts and the bursa. Octopamine also increased the levels of peristaltic and phasic contractions, to the common oviduct, where cAMP in the oviducts, and this effect was blocked by phentolamine. -

Injection Technique 1: Administering Drugs Via the Intramuscular Route
Copyright EMAP Publishing 2018 This article is not for distribution except for journal club use Clinical Practice Keywords Intramuscular injection/ Medicine administration/Absorption Practical procedures This article has been Injection technique double-blind peer reviewed Injection technique 1: administering drugs via the intramuscular route rugs administered by the intra- concerns that nurses are still performing Author Eileen Shepherd is clinical editor muscular (IM) route are depos- outdated and ritualistic practice relating to at Nursing Times. ited into vascular muscle site selection, aspirating back on the syringe Dtissue, which allows for rapid (Greenway, 2014) and skin cleansing. Abstract The intramuscular route allows absorption into the circulation (Dough- for rapid absorption of drugs into the erty and Lister, 2015; Ogston-Tuck, 2014). Site selection circulation. Using the correct injection Complications of poorly performed IM Four muscle sites are recommended for IM technique and selecting the correct site injection include: administration: will minimise the risk of complications. l Pain – strategies to reduce this are l Vastus lateris; outlined in Box 1; l Rectus femoris Citation Shepherd E (2018) Injection l Bleeding; l Deltoid; technique 1: administering drugs via l Abscess formation; l Ventrogluteal (Fig 1, Table 1). the intramuscular route. Nursing Times l Cellulitis; Traditionally the dorsogluteal (DG) [online]; 114: 8, 23-25. l Muscle fibrosis; muscle was used for IM injections but this l Injuries to nerves and blood vessels muscle is in close proximity to a major (Small, 2004); blood vessel and nerves, with sciatic nerve l Inadvertent intravenous (IV) access. injury a recognised complication (Small, These complications can be avoided if 2004). -

Self-Administering a Vitamin B12 Injection
Self-administering a Vitamin B12 injection This is usually given as an intramuscular injection, every 2-3 months. Alternatives to an intramuscular injection are: Oral Vitamin B12 at a dose of at least 1000mcg per day. • Available as tablets or a spray. They can be bought over the counter and available at most health food stores and pharmacies. • Oral Vitamin B12 is not recommended if: - If you have a bowel condition such as inflammatory bowel disease, Coeliac disease, small bowel overgrowth, bile acid malabsorption and short bowel (you will require injections) - You require an initial loading of B12 (soon after diagnosis) It is important to monitor your symptoms if you change to oral B12. If symptoms return, then the oral/sublingual dose can be increased to 2000mcg or you may need to consider starting back on injections. https://www.hollandandbarrett.com/shop/product/betteryou-pure-energy-b12-boost-oral-spray-60099160?skuid=099160 https://www.hollandandbarrett.com/search?query=%20Vitamin%20B12%20Tablets&utm_medium=cpc&utm_source=google&isSearch=true# gclid=EAIaIQobChMIh5nLgOH26AIVxLTtCh0JIA_GEAAYASAAEgJL-fD_BwE&gclsrc=aw.ds Subcutaneous (SC) injection; this is off-licence but still effective. It is considered an easier method of administration. It is how insulin and blood thinning medication are usually self-administered. Equipment needed to self-inject - Prescribed medicine - 1 syringe (2 ml) - 2 needles (1 for drawing up the drug and 1 for administration – you can use the same size needle for both). o For an IM injection; the needle gauge should be 19-25. The needle length is 1- 1 ½ inches (up to 3 inches for larger adults) o For a SC injection; the needle gauge should be 25-27. -

Can Hepatic Coma Be Caused by a Reduction of Brain Noradrenaline Or Dopamine?
Gut: first published as 10.1136/gut.18.9.688 on 1 September 1977. Downloaded from Gut, 1977, 18, 688-691 Can hepatic coma be caused by a reduction of brain noradrenaline or dopamine? L. ZIEVE AND R. L. OLSEN From the Department ofMedicine, Minneapolis Veterans Hospital, University of Minnesota, Minneapolis, and Department of Chemistry, Hamline University, St. Paul, Minnesota, USA SUMMARY Intraventricular infusions of octopamine which raised brain octopamine concentrations more than 20 000-fold resulted in reductions in brain noradrenaline and dopamine by as much as 90% without affecting the alertness or activity of normal rats. As this reduction of brain catechol- amines is much greater than any reported in hepatic coma, we do not believe that values observed in experimental hepatic failure have aetiological significance for the encephalopathy that ensues. Though catecholaminergic nerve terminals represent dopamine and noradrenaline had no discernible only a small proportion of brain synapses (Snyder et effect on the state of alertness of the animals. al., 1973), the reduction in brain dopamine or nor- adrenaline by the accumulation of false neuro- Methods transmitterssuch as octopamine or of aromaticamino acids such as phenylalanine or tyrosine has been ANIMALS suggested as a cause of hepatic coma (Fischer and Male Sprague-Dawley rats weighing between 300 and Baldessarini, 1971; Dodsworth et al., 1974; Fischer 350 were g prepared by the method of Peterson and http://gut.bmj.com/ and Baldessarini, 1975; Munro et al., 1975). The Sparber (1974) for intraventricular infusions of following data have been cited in support of this octopamine. Rats of 250-400 g were used as controls. -

Structure-Cytotoxicity Relationship Profile of 13 Synthetic Cathinones In
Neurotoxicology 75 (2019) 158–173 Contents lists available at ScienceDirect Neurotoxicology journal homepage: www.elsevier.com/locate/neuro Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells T ⁎ Jorge Soaresa, , Vera Marisa Costaa, Helena Gasparb,c, Susana Santosd,e, Maria de Lourdes Bastosa, ⁎ Félix Carvalhoa, João Paulo Capelaa,f, a UCIBIO, REQUIMTE (Rede de Química e Tecnologia), Laboratório de Toxicologia, Departamento de Ciências Biológicas, Faculdade de Farmácia, Universidade do Porto, Portugal b BioISI – Instituto de Biossistemas e Ciências Integrativas, Faculdade de Ciências, Universidade de Lisboa, Portugal c MARE - Centro de Ciências do Mar e do Ambiente, Escola Superior de Turismo e Tecnologia do Mar, Instituto Politécnico de Leiria, Portugal d Centro de Química e Bioquímica (CQB), Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Portugal e Centro de Química Estrutural, Faculdade de Ciências, Universidade de Lisboa, Portugal f FP-ENAS (Unidade de Investigação UFP em Energia, Ambiente e Saúde), CEBIMED (Centro de Estudos em Biomedicina), Faculdade de Ciências da Saúde, Universidade Fernando Pessoa, Portugal ARTICLE INFO ABSTRACT Keywords: Synthetic cathinones also known as β-keto amphetamines are a new group of recreational designer drugs. We Synthetic cathinones aimed to evaluate the cytotoxic potential of thirteen cathinones lacking the methylenedioxy ring and establish a Classical amphetamines putative structure-toxicity profile using differentiated SH-SY5Y cells, as well as to compare their toxicity to that Cytotoxicity of amphetamine (AMPH) and methamphetamine (METH). Cytotoxicity assays [mitochondrial 3-(4,5-dimethyl-2- SH-SY5Y cells thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction and lysosomal neutral red (NR) uptake] per- Structure-toxicity relationship formed after a 24-h or a 48-h exposure revealed for all tested drugs a concentration-dependent toxicity. -

The Role of Epinephrine in the Treatment of Anaphylaxis Anne K
The Role of Epinephrine in the Treatment of Anaphylaxis Anne K. Ellis, MD, and James H. Day, MD, FRCP(C) Address Beneficial Effects of Epinephrine Division of Allergy, Kingston General Hospital, 76 Stuart Street, in Anaphylaxis Kingston, ON K7L 2V7, Canada. Epinephrine is both an alpha (α) and a beta (β) adrener- E-mail: [email protected] gic receptor agonist. Through α-adrenergic stimulation, Current Allergy and Asthma Reports 2003, 3:11–14 epinephrine increases peripheral vascular resistance, Current Science Inc. ISSN 1529-7322 Copyright © 2003 by Current Science Inc. improving blood pressure and coronary artery perfusion, reversing peripheral vasodilation, and decreasing angioedema and urticaria [5]. β-1 adrenergic stimulation Epinephrine is the cornerstone of anaphylaxis manage- has positive inotropic and chronotropic effects on the ment. Its administration should be immediate upon evi- myocardium, and β-2 adrenergic effects include bron- dence of the occurrence of anaphylaxis. Delays in chodilation [6]. β-adrenergic receptors also increase administration may be fatal. The most appropriate admin- intracellular cyclic adenosine monophosphate (cAMP) istration is 0.3 to 0.5 mL of 1:1000 dilution intramuscu- production in mast cells and basophils, which inhibits larly for adults and 0.01 mg/kg for children, given in the further inflammatory mediator release [7,8]. lateral thigh. Patients with known anaphylactic reactivity should be prescribed an epinephrine auto-injector to be carried at all times for treatment of potential recur- Dosage and Routes of Administration rences. Education of the patient or parent regarding the for Epinephrine proper use of this tool is paramount. Some discrepancy exists in the literature concerning the appropriate dosage of epinephrine for anaphylaxis. -

Guideline (S2k) on Acute Therapy and Management of Anaphylaxis: 2021 Update
guidelines Allergo J Int (2021) 30:1–25 https://doi.org/10.1007/s40629-020-00158-y Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update S2k-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Medical Association of German Allergologists (AeDA), the Society of Pediatric Allergology and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Society for Neonatology and Pediatric Intensive Care (GNPI), the German Society of Dermatology (DDG), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Respiratory Society (DGP), the patient organization German Allergy and Asthma Association (DAAB), the German Working Group of Anaphylaxis Training and Education (AGATE) Johannes Ring · Kirsten Beyer · Tilo Biedermann · Andreas Bircher · Matthias Fischer · Thomas Fuchs · Axel Heller · Florian Hoffmann · Isidor Huttegger · Thilo Jakob · Ludger Klimek · Matthias V. Kopp · Claudia Kugler · Lars Lange · Oliver Pfaar · Ernst Rietschel · Franziska Rueff · Sabine Schnadt · Roland Seifert · Britta Stöcker · Regina Treudler · Christian Vogelberg · Thomas Werfel · Margitta Worm · Helmut Sitter · Knut Brockow Published online: 28 January 2021 © The Author(s) 2021 Keywords Anaphylaxis · Food allergy · Drug LT Leukotriene -

Efficacy/Risk Profile of Triamcinolone Acetonide in Severe Asthma
ARTICLE IN PRESS Respiratory Medicine CME (2008) 1, 111–115 respiratory MEDICINE CME CASE REPORT Efficacy/risk profile of triamcinolone acetonide in severe asthma: Lessons from one case study Cesar Picadoà Servei de Pneumologia, Hospital Clinic, Universitat de Barcelona, Villarroel 170, 08036 Barcelona, Spain Received 26 November 2007; accepted 22 January 2008 KEYWORDS Summary Insulin; Some prednisone-resistant asthma patients respond to intramuscular triamcinolone Refractory asthma; acetonide (TA). The use of TA has been questioned on the basis of its potential toxicity. Severe asthma; TA’s ratio of clinical efficacy to systemic effects compared with oral prednisone has not Steroid-dependent been clearly defined. asthma; We report the case of a prednisone-insensitive severe asthma patient with insulin- Triamcinolone dependent diabetes, hypertension and diabetic retinopathy treated with repeated sessions acetonide of laser therapy. By daily monitoring the needs of insulin doses we compared the systemic effects of prednisone and TA. The analysis of the balance between systemic effects (insulin doses) and beneficial effects (PEF values) show that TA has a better benefit/risk profile than prednisone. The patient has been followed up for 3 years and has received injections of TA repeated at intervals ranging from 21 to 60 days according to patient response and the evolution of PEF and clinical symptoms. During this period the patient lost 3 kg of weight, while presenting increased bone mineral density, normalized arterial tension, and improved proliferative diabetic retinopathy. The present case report shows that TA has a better benefit/risk profile than prednisone. TA can be a valuable alternative in the control of some patients with severe prednisone- resistant asthma. -

How to Administer Intramuscular, Intradermal, and Intranasal Influenza Vaccines
How to Administer Intramuscular, Intradermal, and Intranasal Influenza Vaccines Intramuscular injection (IM) Intradermal administration (ID) Intranasal administration (NAS) Inactivated Influenza Vaccines (IIV), including Inactivated Influenza Vaccine (IIV) Live Attenuated Influenza Vaccine (LAIV) recombinant hemagglutinin influenza vaccine (RIV3) 1 Gently shake the microinjection system before 1 FluMist (LAIV) is for intranasal administration 1 Use a needle long enough to reach deep into administering the vaccine. only. Do not inject FluMist. the muscle. Infants age 6 through 11 mos: 1"; 1 through 2 yrs: 1–1¼"; children and adults 2 Hold the system by placing the 2 Remove rubber tip protector. Do not remove 3 yrs and older: 1–1½". thumb and middle finger on dose-divider clip at the other end of the sprayer. the finger pads; the index finger 2 With your left hand*, bunch up the muscle. should remain free. 3 With the patient in an upright position, place the tip just inside the nostril 3 With your right hand*, insert the needle at a 3 Insert the needle perpendicular to the skin, to ensure LAIV is delivered into 90° angle to the skin with a quick thrust. in the region of the deltoid, in a short, quick the nose. The patient should movement. breathe normally. 4 Push down on the plunger and inject the entire contents of the syringe. There is no need to 4 Once the needle has been 4 With a single motion, depress plunger as aspirate. inserted, maintain light pressure rapidly as possible until the dose-divider clip on the surface of the skin and prevents you from going further. -

Mutasem Rawas.Qalaji
FORMULATION AND ASSESSMENT OF FAST- DISINTEGRATING SUBLINGUAL EPINEPHRINE TABLETS FOR TI.IE POTENTIAI- EMERGENCY TREATMENT OF ANAPHYLAXIS By MUTASEM RAWAS.QALAJI A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY FACULTY OF PHARMACY UNIVERSITY OF MANITOBA WINNIPEG, MANITOBA, CANADA JUNE, 2006 THE UNIVERSITY OF MANITOBA FACULTY OF GRADUATE STUDIES COPYRIGHT PERMISSION Formulation and Assessment of Fast-Disintegrating Sublingual Epinephrine Tablets for the Potential Emergency Treatment of Anaphylaxis By Mutasem Rawas-Qalaji A Thesis/Practicum submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fïlfitlment of the requirement of the degree of Doctor of Philosophy Mutasem Rawas-Qalaji @ 2006 Permission has been granted to the Library of the University of Manitoba to lend or sell copies of this thesis/practicum, to the National Library of Canada to microfilm this thesis and to lend or sell copies of the film, and to University Microfilms Inc. to publish an abstract of this thesis/practicum. This reproduction or copy of this thesis has been made available by authority of the copyright owner solely for the purpose of private study and research, and may only be reproduced and copied as permitted by copyright laws or with express written authorization from the copyright o\ilner. DEDICATION To my father Mohamad Rawas-ealaji and my mother wedad Duqsi (in memoriam) lwould like to dedicate this work. ABSTRACT Objectives; To formulate, characterize, and evaluate the stability of sublingual (sL) epinephrine (E) tablets that are bioequivatent to E 0.3 mg intramuscular injection (lM) for the feasibility of SL E administration for the potential out-of-hospital emergency treatment of anaphylaxis.