V Nucleoside/Nucleotide RT Inhibitor (NRTI) Resistance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment-Drugs
© National HIV Curriculum PDF created September 23, 2021, 9:14 am Stavudine (Zerit) Table of Contents Stavudine Zerit Summary Drug Summary Key Clinical Trials Resistance Key Drug Interactions Drug Summary Stavudine, an early nucleoside reverse transcriptase inhibitor (NRTI), was used as part of combination antiretroviral therapy for years, but now has become obsolete and replaced by better-tolerated and safer options. Stavudine poses risk of serious toxicity, including peripheral neuropathy (which can be permanent), pancreatitis, lipoatrophy, and lactic acidosis. Fatal and nonfatal cases of pancreatitis and lactic acidosis have been reported, especially when stavudine was combined with didanosine. According to the Adult and Adolescent ARV Guidelines, stavudine is no longer recommended for the treatment of HIV infection due to potential severe toxicity. Further, all persons currently taking stavudine should be strongly encouraged to switch to a safer medication. The sale and distribution of all strengths of stavudine will be discontinued and removed from the market in the United States in 2020. Key Clinical Trials Stavudine was studied for treatment-naïve patients as part of triple therapy, such as with lamivudine plus indinavir [START I], lamivudine plus lopinavir-ritonavir [M98-863], and lamivudine plus efavirenz [DART II]. Several studies demonstrated benefits of switching stavudine to newer NRTI agents, such as tenofovir disoproxil fumarate; the switch led to decreased rates of metabolic complications and mitochondrial toxicity [903E, SNAP, and ACTG 5142]. Resistance For a listing of the most common clinically significant mutations associated with stavudine (d4T) resistance, see the NRTI Resistance Notes on the Stanford University HIV Drug Resistance Database. Page 1/2 Key Drug Interactions For complete information on stavudine-related drug interactions, see the Drug Interactions section in the Stavudine (Zerit) Prescribing Information. -

Eparate Formulations According to the Prescribed Dosing Recommendations for These Products
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Lamivudine/Zidovudine Teva 150 mg/300 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 150 mg lamivudine and 300 mg zidovudine. For the full list of excipients see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet White, capsule shaped, biconvex, film-coated scored tablet – engraved with “L/Z” on one side and “150/300” on the other side. The tablet can be divided into equal halves. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Lamivudine/Zidovudine Teva is indicated in antiretroviral combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection (see section 4.2). 4.2 Posology and method of administration Therapy should be initiated by a physician experienced in the management of HIV infection. Lamivudine/Zidovudine Teva may be administered with or without food. To ensure administration of the entire dose, the tablet(s) should ideally be swallowed without crushing. For patients who are unable to swallow tablets, tablets may be crushed and added to a small amount of semi-solid food or liquid, all of which should be consumed immediately (see section 5.2). Adults and adolescents weighing at least 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one tablet twice daily. Children weighing between 21 kg and 30 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken in the morning and one whole tablet taken in the evening. Children weighing from 14 kg to 21 kg: the recommended oral dose of Lamivudine/Zidovudine Teva is one-half tablet taken twice daily. -
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
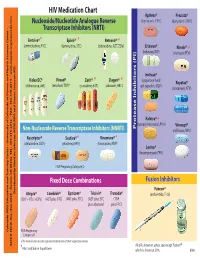
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Product Monograph for CELSENTRI
PRODUCT MONOGRAPH PrCELSENTRI maraviroc Tablets 150 and 300 mg CCR5 antagonist ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: July 05, 2019 Submission Control No: 226222 © 2019 ViiV Healthcare group of companies or its licensor. Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 60 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION.........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS....................................................................................................9 DRUG INTERACTIONS ..................................................................................................19 DOSAGE AND ADMINISTRATION..............................................................................28 OVERDOSAGE ................................................................................................................31 ACTION AND CLINICAL PHARMACOLOGY ............................................................31 STORAGE AND STABILITY..........................................................................................36 -

Design and Evaluation of 5•²-O-Dicarboxylic And
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2014 DESIGN AND EVALUATION OF 5′-O-DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES Bhanu Priya Pemmaraju Venkata University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Pemmaraju Venkata, Bhanu Priya, "DESIGN AND EVALUATION OF 5′-O-DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES" (2014). Open Access Master's Theses. Paper 474. https://digitalcommons.uri.edu/theses/474 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. DESIGN AND EVALUATION OF 5′-O- DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES BY BHANU PRIYA, PEMMARAJU VENKATA A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE MASTER’S DEGREE IN BIOMEDICAL AND PHARMACEUTICAL SCIENCES UNIVERSITY OF RHODE ISLAND 2014 MASTER OF SCIENCE THESIS OF BHANU PRIYA, PEMMARAJU VENKATA APPROVED: Thesis Committee: Major Professor Keykavous Parang Roberta King Stephen Kogut Geoffrey D. Bothun Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT 2′,3′-Dideoxynucleoside (ddNs) analogs are the most widely used anti-HIV drugs in the market. Even though these drugs display very potent activities, they have a number of limitations when are used as therapeutic agents. The primary problem associated with ddNs is significant toxicity, such as neuropathy and bone marrow suppression. -

DESCOVY, and Upon Diagnosis of These Highlights Do Not Include All the Information Needed to Use Any Other Sexually Transmitted Infections (Stis)
HIGHLIGHTS OF PRESCRIBING INFORMATION once every 3 months while taking DESCOVY, and upon diagnosis of These highlights do not include all the information needed to use any other sexually transmitted infections (STIs). (2.2) DESCOVY safely and effectively. See full prescribing information • Recommended dosage: for DESCOVY. • Treatment of HIV-1 Infection: One tablet taken once daily with or ® without food in patients with body weight at least 25 kg. (2.3) DESCOVY (emtricitabine and tenofovir alafenamide) tablets, for • HIV-1 PrEP: One tablet taken once daily with or without food in oral use individuals with body weight at least 35 kg. (2.4) Initial U.S. Approval: 2015 • Renal impairment: DESCOVY is not recommended in individuals with WARNING: POST-TREATMENT ACUTE EXACERBATION OF estimated creatinine clearance below 30 mL per minute. (2.5) HEPATITIS B and RISK OF DRUG RESISTANCE WITH USE ----------------------DOSAGE FORMS AND STRENGTHS-------------------- OF DESCOVY FOR HIV-1 PRE-EXPOSURE PROPHYLAXIS Tablets: 200 mg of FTC and 25 mg of TAF (3) (PrEP) IN UNDIAGNOSED EARLY HIV-1 INFECTION See full prescribing information for complete boxed warning. -------------------------------CONTRAINDICATIONS------------------------------ DESCOVY for HIV-1 PrEP is contraindicated in individuals with Severe acute exacerbations of hepatitis B (HBV) have been unknown or positive HIV-1 status. (4) reported in HBV-infected individuals who have discontinued products containing emtricitabine (FTC) and/or tenofovir -----------------------WARNINGS AND PRECAUTIONS----------------------- disoproxil fumarate (TDF), and may occur with • Comprehensive management to reduce the risk of sexually discontinuation of DESCOVY. Hepatic function should be transmitted infections (STIs), including HIV-1, when DESCOVY is monitored closely in these individuals. -

Lamivudine in Combination with Zidovudine, Stavudine, Or Didanosine in Patients with HIV-1 Infection
Lamivudine in combination with zidovudine, stavudine, or didanosine in patients with HIV-1 infection. A randomized, double-blind, placebo-controlled trial Daniel R. Kuritzkes*, Ian Marschner†, Victoria A. Johnson‡, Roland Bassett†, Joseph J. Eron§, Margaret A. FischlII, Robert L. Murphy¶, Kenneth Fife**, Janine Maenza††, Mary E. Rosandich*, Dawn Bell‡‡, Ken Wood§§, Jean-Pierre Sommadossi‡, Carla PettinelliII II and the National Institute of Allergy and Infectious Disease AIDS Clinical Trials Group Protocol 306 Investigators Objective: To study the antiviral activity of lamivudine (3TC) plus zidovudine (ZDV), didanosine (ddI), or stavudine (d4T). Design: Randomized, placebo-controlled, partially double-blinded multicenter study. Setting: Adult AIDS Clinical Trials Units. Patients: Treatment-naive HIV-infected adults with 200–600 × 106 CD4 T lymphocytes/l. Interventions: Patients were openly randomized to a d4T or a ddI limb, then randomized in a blinded manner to receive: d4T (80 mg/day), d4T plus 3TC (300 mg/day), or ZDV (600 mg/day) plus 3TC, with matching placebos; or ddI (400 mg/day), ddI plus 3TC (300 mg/day), or ZDV (600 mg/day) plus 3TC, with matching placebos. After 24 weeks 3TC was added for patients assigned to the monotherapy arms. Main outcome measure: The reduction in plasma HIV-1 RNA level at weeks 24 and 48. From the *University of Colorado Health Sciences Center and Veterans Affairs Medical Center, Denver, Colorado, the †Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, Massachusetts, the -

(12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson Et Al
US 20080241135A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson et al. (43) Pub. Date: Oct. 2, 2008 (54) METHODS FOR REDUCINGVIRAL LOAD IN 60/711,528, filed on Aug. 26, 2005, provisional appli HIV-1-INFECTED PATIENTS cation No. 60/715,619, filed on Sep. 9, 2005. (75) Inventors: William C. Olson, Ossining, NY Publication Classification (US); Paul J. Maddon, Scarsdale, NY (US); Daniel C. Pevear, (51) Int. Cl. Medford, MA (US); Robert J. A 6LX 39/395 (2006.01) Israel, Suffern, NY (US); Jose D. A6IP 43/00 (2006.01) Murga, Rosedale, NY (US) (52) U.S. Cl. ..................................................... 424/133.1 Correspondence Address: (57) ABSTRACT COOPER & DUNHAM, LLP 1185 AVENUE OF THE AMERICAS This method provides a method for reducing HIV-1 viral load NEW YORK, NY 10036 in an HIV-1-infected human subject which comprises admin istering to the subject at a predefined interval effective HIV-1 (73) Assignee: Progenics Pharmaceuticals, Inc. viral load-reducing doses of (a) a humanized antibody desig nated PRO 140, or of (b) an anti-CCR5 receptor monoclonal (21) Appl. No.: 11/894,762 antibody. This invention also provides a method for inhibiting in a human Subject the onset or progression of an HIV-1- (22) Filed: Aug. 20, 2007 associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5"CD4" target cells in the Related U.S. Application Data Subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the (63) Continuation of application No. -

DESCOVY® Formulary Monograph
DESCOVY® Formulary Monograph Click here for full Prescribing Information for DESCOVY, including BOXED WARNING. TABLE OF CONTENTS EXECUTIVE SUMMARY BOXED WARNING . 3 Indication . 3 Dosage and administration . 3 Adverse reactions . 4 Manufacturer . 4 Dosage forms and how supplied . 4 Wholesale acquisition cost (WAC) . 4 FDA approval date . 4 Clinical data summary . 5 HIV DISEASE STATE OVERVIEW The HIV patient profile . 7 The importance of early treatment . 7 Treatment overview . 8 DESCOVY SUMMARY OF PRODUCT INFORMATION BOXED WARNING . 9 Indication and usage . 9 Dosage and administration . 9 Product description and dosage forms . 10 Warnings and precautions . 10 Lactic acidosis/severe hepatomegaly with steatosis . 10 Severe acute exacerbation of hepatitis B in patients coinfected with HIV-1 and HBV . 10 Fat redistribution . 11 Immune reconstitution syndrome . 11 New onset or worsening renal impairment . 11 Bone loss and mineralization defects . 12 Adverse reactions . 12 Adverse reactions in clinical trials of FTC+TAF with EVG+COBI in treatment-naïve adults with HIV-1 infection . 12 Renal laboratory tests . 13 Bone mineral density effects . 13 Adverse reactions in clinical trials in pediatric patients with HIV-1 infection . 13 Drug interactions . 14 Potential for other drugs to affect one or more components of DESCOVY . 14 Drugs affecting renal function . 14 Established and other potentially significant drug interactions . 14 Drugs without clinically significant interactions with DESCOVY . 15 Click here for full Prescribing Information for DESCOVY, including BOXED WARNING. 1 TABLE OF CONTENTS DESCOVY SUMMARY OF PRODUCT INFORMATION (cont .) Use in specific populations . 15 Pregnancy . 15 Pregnancy exposure registry . 15 Risk summary . 15 Human data . 15 Lactation . 16 Risk summary . -

Recent Advances in Antiviral Therapy J Clin Pathol: First Published As 10.1136/Jcp.52.2.89 on 1 February 1999
J Clin Pathol 1999;52:89–94 89 Recent advances in antiviral therapy J Clin Pathol: first published as 10.1136/jcp.52.2.89 on 1 February 1999. Downloaded from Derek Kinchington Abstract indicated that using a combination of drugs In the early 1980s many institutions in might overcome this problem. The only Britain were seriously considering available drugs during the late 1980s were two whether there was a need for specialist other nucleotide reverse transcriptase inhibi- departments of virology. The arrival of tors (NRTI) which also targeted HIV reverse HIV changed that perception and since transcriptase (HIV-RT): 2',3'-dideoxycytidine then virology and antiviral chemotherapy (ddC) and 2',3'-dideoxyinosine (ddI).56 In have become two very active areas of bio- vitro combination studies gave surprising medical research. Cloning and sequencing results: those viruses that became highly resist- have provided tools to identify viral en- ant to ZDV remained sensitive to both ddC zymes and have brought the day of the and ddI.7 Furthermore, neither cross resistance “designer drug” nearer to reality. At the nor interference between the drugs was an other end of the spectrum of drug discov- issue, and subsequent clinical experience ery, huge numbers of compounds for showed that patients benefited when these two screening can now be generated by combi- compounds were used in combination with natorial chemistry. The impetus to find ZDV.8 It was also found by in vitro studies that drugs eVective against HIV has also virus isolated from patients on long term ZDV stimulated research into novel treatments monotherapy had become insensitive to ZDV, for other virus infections including her- but regained sensitivity when these patients pesvirus, respiratory infections, and were switched to ddI monotherapy. -

Guidelines for the Use of Antiretroviral Agents in Adults and Adolescent Living With
Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ AdultandAdolescentGL.pdf. Accessed [insert date] [insert page number, table number, etc. if applicable] It is emphasized that concepts relevant to HIV management evolve rapidly. The Panel has a mechanism to update recommendations on a regular basis, and the most recent information is available on the HIVinfo Web site (http://hivinfo.nih.gov). What’s New in the Guidelines? August 16, 2021 Hepatitis C Virus/HIV Coinfection • Table 18 of this section has been updated to include recommendations regarding concomitant use of fostemsavir or long acting cabotegravir plus rilpivirine with different hepatitis C treatment regimens. June 3, 2021 What to Start • Since the release of the last guidelines, updated data from the Botswana Tsepamo study have shown that the prevalence of neural tube defects (NTD) associated with dolutegravir (DTG) use during conception is much lower than previously reported. Based on these new data, the Panel now recommends that a DTG-based regimen can be prescribed for most people with HIV who are of childbearing potential. Before initiating a DTG-based regimen, clinicians should discuss the risks and benefits of using DTG with persons of childbearing potential, to allow them to make an informed decision.