Guidelines for the Use of SI Units in Technical Writing and Presentations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Learner's Guide to SI Units and Their Conversion
A Learner's Guide to SI Units and their Conversion October 2004 Workbook for students Learner's Guide to SI Units and their Conversion their and Units SI to Guide Learner's Edexcel A London Qualifications is one of the leading examining and awarding bodies in the UK and throughout the world. It incorporates all the qualifications previously awarded under the Edexcel and BTEC brand. We provide a wide range of qualifications including general (academic), vocational, occupational and specific programmes for employers. Through a network of UK and overseas offices, our centres receive the support they need to help them deliver their education and training programmes to learners. For further information please call Customer Services on 0870 240 9800, or visit our website at www.edexcel.org.uk Authorised by Jim Dobson Prepared by Sarah Harrison All the material in this publication is copyright © London Qualifications Limited 2004 CONTENTS Introduction 1 What are units? 2 Operations with units 5 Submultiple and multiple units 9 Conversion of units 11 Conversion examples and exercises 13 Length 13 Area 14 Volume 15 Mass 16 Time 17 Temperature 18 Density 19 Force 20 Stress and pressure 21 Answers to exercises 23 Introduction One of the important areas where Science and Technology students need support is in the conversion of units. This booklet is designed to be useful for students in all Science, Technology and Engineering subjects. This booklet has been produced to: S introduce students to SI base and derived units and S help students with the conversion of multiple and sub-multiple units to SI base and derived units. -

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -
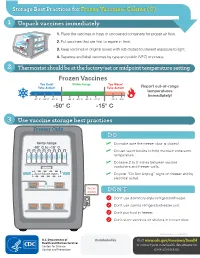
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -
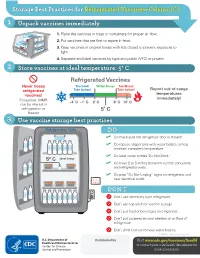
Storage Best Practices for Refrigerated Vaccines–Celsius (C)
Storage Best Practices for Refrigerated Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Store vaccines at ideal temperature: 5° C Refrigerated Vaccines Never freeze Too Cold! Within Range Too Warm! refrigerated Take Action! Take Action! Report out-of-range vaccines! temperatures Exception: MMR immediately! -4˚ C -1˚ C 2˚ C 8˚ C 10˚ C can be stored in refrigerator or 5˚ C freezer 3 Use vaccine storage best practices Refrigerator Only DO ✓ Do make sure the refrigerator door is closed! ✓ Do replace crisper bins with water bottles to help maintain consistent temperature. ✓ Do label water bottles "Do Not Drink." 5° C ideal temp ✓ Do leave 2 to 3 inches between vaccine containers and refrigerator walls. ✓ Do post “Do Not Unplug” signs on refrigerator and do not near electrical outlet. unplug DON’T ; Don’t use dormitory-style refrigerator. ; Don’t use top shelf for vaccine storage. ; Don’t put food or beverages in refrigerator. ; Don’t put vaccines on door shelves or on floor of refrigerator. Don’t drink from or remove water bottles. ; CS243541-C Revision February 2018 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Can you find at least 8 things that are wrong with vaccine storage in this refrigerator? 5° C ideal temp 2 When unpacking vaccines, why is it important to put the first-to-expire in the front? A. -

Worksheet 2 the Metric System and SI Units
Unit Conversions Worksheet 2 The Metric System and SI Units 1 © MathTutorDVD.com Here is a helpful list of all the prefixes and their exponents and abbreviations: 109 giga G one billion 106 mega M one million 103 kilo k one thousand 102 hecto h one hundred 101 deca da ten 10-1 deci d one tenth 10-2 centi c one hundredth 10-3 milli m one thousandth 10-6 micro µ one millionth 10-9 nano n one billionth 10-12 pico p one trillionth 2 © MathTutorDVD.com 1. Give the abbreviation for each of the SI base units below. a. kilogram b. Kelvin c. meter d. second 2. State what quantities the following SI units are used to measure. a. kg b. m c. s d. K 3. Give the power of 10 that each of the following prefixes represents. a. kilo b. micro c. centi d. deca e. mega 3 © MathTutorDVD.com 4. Write the following numbers in their base units. a. 5 kilometers b. 12 centimeters c. 2 micrograms d. 4.1 megagrams 5. Write the abbreviations for the following measurements. a. 67 micrograms b. 831 kilometers c. 1.2 meters d. 791 megagrams 4 © MathTutorDVD.com 6. Express the following numbers with an appropriate SI prefix (e.g., 5,000 g = 5 kg). a. 7800 m b. 5.0 x 10-6 g c. 7.8 x 106 m d. 1.6 x 10-3 g 7. Convert the following to scientific notation using the base SI unit. a. 4.51 microseconds b. 6700 grams c. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -
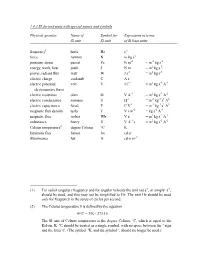
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

The International System of Units (SI)
The International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 330 2008 Edition Barry N. Taylor and Ambler Thompson, Editors NIST SPECIAL PUBLICATION 330 2008 EDITION THE INTERNATIONAL SYSTEM OF UNITS (SI) Editors: Barry N. Taylor Physics Laboratory Ambler Thompson Technology Services National Institute of Standards and Technology Gaithersburg, MD 20899 United States version of the English text of the eighth edition (2006) of the International Bureau of Weights and Measures publication Le Système International d’ Unités (SI) (Supersedes NIST Special Publication 330, 2001 Edition) Issued March 2008 U.S. DEPARTMENT OF COMMERCE, Carlos M. Gutierrez, Secretary NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY, James Turner, Acting Director National Institute of Standards and Technology Special Publication 330, 2008 Edition Natl. Inst. Stand. Technol. Spec. Pub. 330, 2008 Ed., 96 pages (March 2008) CODEN: NSPUE2 WASHINGTON 2008 Foreword The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant system used in science, the SI is rapidly becoming the dominant measurement system used in international commerce. In recognition of this fact and the increasing global nature of the marketplace, the Omnibus Trade and Competitiveness Act of 1988, which changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness, designates “the metric system of measurement as the preferred system of weights and measures for United States trade and commerce.” The definitive international reference on the SI is a booklet published by the International Bureau of Weights and Measures (BIPM, Bureau International des Poids et Mesures) and often referred to as the BIPM SI Brochure. -

Table of Contents
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Dietetic Technician 13 - Trying Out Metric Units 14 - Measuring With Metrics 15 Unit 3 16 - Objective 16 - Suggested Teaching Sequence 16 - Metric-Metric Equivalents 16 - Changing Units at Work 18 Unit 4 19 - Objective 19 - Suggested Teaching Sequence 19 - Selecting and Using Metric Instruments, Tools and Devices 19 - Which Tools for the Job? 20 - Measuring Up The Dietary Department 20 Unit 5 21 - Objective 21 - Suggested Teaching Sequence 21 - Metric-Customary Equivalents 21 - Conversion Table 22 - Any Way You Want It 23 Testing Metric Abilities 24 Answers to Exercises and Test 25 Temperature 26 Tools and Devices List References a::3CENTVI FOR VOCATlONAL I!DUCATION TEACHING AND LEARNING THE METRIC SYSTEM This metric instructional package was designed to meet job-related Unit 2 provide'S the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships.