The Multifaceted Roles of Copper in Cancer: a Trace Metal Element with Dysregulated Metabolism, but Also a Target Or a Bullet for Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Iron Metabolism-Related Genes in Prenatal Development: Insights from Mouse Transgenic Models
G C A T T A C G G C A T genes Review Role of Iron Metabolism-Related Genes in Prenatal Development: Insights from Mouse Transgenic Models Zuzanna Kope´c , Rafał R. Starzy ´nski,Aneta Jo ´nczy , Rafał Mazgaj and Paweł Lipi ´nski* Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, 05-552 Jastrz˛ebiec,Poland; [email protected] (Z.K.); [email protected] (R.R.S.); [email protected] (A.J.); [email protected] (R.M.) * Correspondence: [email protected] Abstract: Iron is an essential nutrient during all stages of mammalian development. Studies car- ried out over the last 20 years have provided important insights into cellular and systemic iron metabolism in adult organisms and led to the deciphering of many molecular details of its regula- tion. However, our knowledge of iron handling in prenatal development has remained remarkably under-appreciated, even though it is critical for the health of both the embryo/fetus and its mother, and has a far-reaching impact in postnatal life. Prenatal development requires a continuous, albeit quantitatively matched with the stage of development, supply of iron to support rapid cell division during embryogenesis in order to meet iron needs for erythropoiesis and to build up hepatic iron stores, (which are the major source of this microelement for the neonate). Here, we provide a concise overview of current knowledge of the role of iron metabolism-related genes in the maintenance of iron homeostasis in pre- and post-implantation development based on studies on transgenic (mainly knock-out) mouse models. -

Happy Fish: a Novel Supplementation Technique to Prevent Iron Deficiency Anemia in Women in Rural Cambodia
Happy Fish: A Novel Supplementation Technique to Prevent Iron Deficiency Anemia in Women in Rural Cambodia by Christopher V. Charles A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Biomedical Science Guelph, Ontario, Canada © Christopher V. Charles, April, 2012 ABSTRACT HAPPY FISH: A NOVEL IRON SUPPLEMENTATION TECHNIQUE TO PREVENT IRON DEFICIENCY ANEMIA IN WOMEN IN RURAL CAMBODIA Christopher V. Charles Advisors: University of Guelph, 2012 Professor Alastair J.S. Summerlee Professor Cate E. Dewey Maternal and child undernutrition are a significant problem in the developing world, with serious consequences for human health and socio-economic development. In Cambodia, 55% of children, 43% of women of reproductive age, and 50% of pregnant women are anemic. Current prevention and control practices rely on supplementation with iron pills or large-scale food fortification, neither of which are affordable or feasible in rural Cambodia. In the study areas, 97% of women did not meet their daily iron requirements. The current research focuses on the design and evaluation of an innovative iron supplementation technique. A culturally acceptable, inexpensive and lightweight iron ingot was designed to resemble a fish species considered lucky in Khmer culture. The ingot, referred to as ‘try sabay’ or ‘happy fish’, was designed to supply iron at a slow, steady rate. Iron leaching was observed in water and soup samples prepared with the iron fish when used concurrently with an acidifier. More than 75% of daily iron requirements can be met with regular use. Its use in the common pot of soup or boiled water provides supplementation to the entire family. -

COX17 (NM 005694) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC210756 COX17 (NM_005694) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: COX17 (NM_005694) Human Tagged ORF Clone Tag: Myc-DDK Symbol: COX17 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC210756 representing NM_005694 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGCCGGGTCTGGTTGACTCAAACCCTGCCCCGCCTGAGTCTCAGGAGAAGAAGCCGCTGAAGCCCTGCT GCGCTTGCCCGGAGACCAAGAAGGCGCGCGATGCGTGTATCATCGAGAAAGGAGAAGAACACTGTGGACA TCTAATTGAGGCCCACAAGGAATGCATGAGAGCCCTAGGATTTAAAATA ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT ACAAGGATGACGACGATAAGGTTTAA Protein Sequence: >RC210756 representing NM_005694 Red=Cloning site Green=Tags(s) MPGLVDSNPAPPESQEKKPLKPCCACPETKKARDACIIEKGEEHCGHLIEAHKECMRALGFKI TRTRPLEQKLISEEDLAANDILDYKDDDDKV Chromatograms: https://cdn.origene.com/chromatograms/mk8114_f02.zip Restriction Sites: SgfI-MluI This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 4 COX17 (NM_005694) Human Tagged ORF Clone – RC210756 Cloning Scheme: Plasmid Map: ACCN: NM_005694 ORF Size: 189 bp This product is -

IRON and ZINC in INFANCY: RESULTS from EXPERIMENTAL TRIALS in SWEDEN and INDONESIA Torbjörn Lind
UMEÅ UNIVERSITY MEDICAL DISSERTATIONS New Series No. 887 – ISSN 0346-6612 – ISBN 91-7305-631-6 From Epidemiology and Public Health Sciences, Department of Public Health and Clinical Medicine & Pediatrics Department of Clinical Sciences Umeå University, 901 87 Umeå, Sweden IRON AND ZINC IN INFANCY: RESULTS FROM EXPERIMENTAL TRIALS IN SWEDEN AND INDONESIA Torbjörn Lind Umeå 2004 Print & Media Copyright Torbjörn Lind Cover illustration: “Mother breastfeeding” Oil on canvas. Yogyakarta 1999. Painter anonymous Printed in Sweden by Print & Media, Umeå 2004 Print & Media “In spite of the spectacular advances in scientific medicine which we have witnessed in the last 20 years, there is still a need for information about a number of fundamental, if quite elementary, matters.” E M Widdowson and C M Spray, 1951 Print & Media Print & Media ABSTRACT Background: Iron and zinc are difficult to provide in sufficient amounts in complementary foods to infants world-wide, resulting in high prevalence of both iron and zinc deficiency. These deficiency states cause anemia, delayed neurodevelopment, impaired growth, and increased susceptibility to infections such as diarrhea and respiratory infections. Design: Two different intervention strategies; reduction of a possible inhibitor of iron and zinc absorption, i.e. phytate, or supplementation with iron and zinc, were applied to two different populations in order to improve iron and zinc nutrition: In a high-income population (Umeå, Sweden), the amount of phytate in commonly consumed infant cereals was reduced. Healthy, term infants (n=300) were at 6 mo of age randomized to phytate-reduced infant cereals, conventional infant cereals, or infant formula and porridge. In a low income population (Purworejo, Indonesia), daily iron and zinc supplementation was given. -

The Effects of Iron Supplementation and Fortification on the Gut Microbiota: a Review
Review The Effects of Iron Supplementation and Fortification on the Gut Microbiota: A Review Emma CL Finlayson-Trick 1 , Jordie AJ Fischer 2,3 , David M Goldfarb 1,3,4 and Crystal D Karakochuk 2,3,* 1 Faculty of Medicine, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; efi[email protected] (E.C.F.-T.); [email protected] (D.M.G.) 2 Department of Food, Nutrition and Health, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; jordie.fi[email protected] 3 British Columbia Children’s Hospital Research Institute, Vancouver, BC V5Z 4H4, Canada 4 Department of Pathology and Laboratory Medicine, BC Children’s and Women’s Hospital and University of British Columbia, Vancouver, BC V6T 1Z7, Canada * Correspondence: [email protected] Received: 30 August 2020; Accepted: 24 September 2020; Published: 26 September 2020 Abstract: Iron supplementation and fortification are used to treat iron deficiency, which is often associated with gastrointestinal conditions, such as inflammatory bowel disease and colorectal cancer. Within the gut, commensal bacteria contribute to maintaining systemic iron homeostasis. Disturbances that lead to excess iron promote the replication and virulence of enteric pathogens. Consequently, research has been interested in better understanding the effects of iron supplementation and fortification on gut bacterial composition and overall gut health. While animal and human trials have shown seemingly conflicting results, these studies emphasize how numerous factors influence gut microbial composition. Understanding how different iron formulations and doses impact specific bacteria will improve the outcomes of iron supplementation and fortification in humans. Furthermore, discerning the nuances of iron supplementation and fortification will benefit subpopulations that currently do not respond well to treatment. -
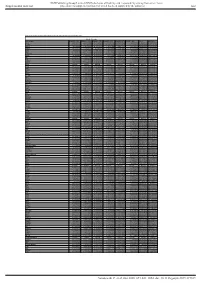
Supl Table 1 for Pdf.Xlsx
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Table S1. Proteomic analysis of liver tissues from 4-months old control and LPTENKO mice. Soluble Fraction Gene names LOG2FC CTL1 LOG2FC CTL2 LOG2FC CTL3 LOG2FC KO1 LOG2FC KO2 LOG2FC KO3 t-test adj Pvalue Aass 0.081686519 -0.097912098 0.016225579 -1.135271836 -0.860535624 -1.263037541 0.0011157 1.206072068 Acsm5 -0.125220746 0.071090866 0.054129881 -0.780692107 -0.882155692 -1.158378647 0.00189031 1.021713016 Gstm2 -0.12707966 0.572554941 -0.445475281 1.952813994 1.856518122 1.561376671 0.00517664 1.865316016 Gstm1 -0.029147714 0.298593425 -0.26944571 0.983159098 0.872028945 0.786125509 0.00721356 1.949464926 Fasn 0.08403202 -0.214149498 0.130117478 1.052480559 0.779734519 1.229308218 0.00383637 0.829422353 Upb1 -0.112438784 -0.137014769 0.249453553 -1.297732445 -1.181999331 -1.495303666 0.00102373 0.184442034 Selenbp2 0.266508271 0.084791964 -0.351300235 -2.040647809 -2.608780781 -2.039865739 0.00107121 0.165425127 Thrsp -0.15001075 0.177999342 -0.027988592 1.54283307 1.603048661 0.927443822 0.00453549 0.612858263 Hyi 0.142635733 -0.183013091 0.040377359 1.325929636 1.119934412 1.181313897 0.00044587 0.053553518 Eci1 -0.119041811 -0.014846366 0.133888177 -0.599970385 -0.555547972 -1.191875541 0.02292305 2.477981171 Aldh1a7 -0.095682449 -0.017781922 0.113464372 0.653424862 0.931724091 1.110750381 0.00356922 0.350756574 Pebp1 -0.06292058 -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Microrna Regulation During Neuroinflammation: from Junk DNA to a Phd Thesis
microRNA regulation during neuroinflammation: from junk DNA to a PhD thesis Camille A. Juźwik A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Neurological Sciences Integrated Program in Neuroscience Department of Neurology and Neurosurgery - McGill University 3801 University Street - Montreal, QC, H3A 2B4, Canada March 2019 © Camille A. Juźwik, 2019 All rights reserved Table of Contents 1 Forward………..……………………………………………………………. 5 1.1 Abstract………………………………………………………………………....................... 5 1.2 Résumé………………………………………………………………………....................... 6 1.3 Acknowledgments…………………………………………………………......................... 8 1.4 Preface and Author Contributions.…...……………………………………………........... 10 1.5 List of Figures and Tables…………………………………………………........................ 12 1.6 List of Abbreviations…….…………………………………………………........................ 15 1.7 List of Publications...…………………………………………………………...................... 17 2 Chapter 1. Introduction…………………………………………………... 19 2.1 General Introduction………………………………………………………………………. 19 2.2 Multiple Sclerosis……..…………………………………………………………………… 22 2.2.1 White matter lesions: axonal pathology….…………………………….............................................................. 24 2.2.2 Gray matter lesions: soma pathology ……….................................................................................................. 25 2.2.3 Experimental autoimmune encephalomyelitis…………………………………….............................................. 27 2.2.4 Not all -

Tumor-Associated NADH Oxidase (Tnox)-NAD+-Sirtuin 1 Axis Contributes to Oxaliplatin-Induced Apoptosis of Gastric Cancer Cells
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 9), pp: 15338-15348 Research Paper Tumor-associated NADH oxidase (tNOX)-NAD+-sirtuin 1 axis contributes to oxaliplatin-induced apoptosis of gastric cancer cells Huei-Yu Chen1, Hsiao-Ling Cheng1, Yi-Hui Lee1, Tien-Ming Yuan1,2, Shi-Wen Chen2, You-Yu Lin1, Pin Ju Chueh1,3,4,5 1Institute of Biomedical Sciences, National Chung Hsing University, Taichung, 40227, Taiwan 2Department of Surgery, Feng-Yuan Hospital, Ministry of Health and Welfare, Taichung 42055, Taiwan 3Graduate Institute of Basic Medicine, China Medical University, Taichung, 40402, Taiwan 4Department of Medical Research, China Medical University Hospital, Taichung, 40402, Taiwan 5Department of Biotechnology, Asia University, Taichung, 41354, Taiwan Correspondence to: Pin Ju Chueh, email: [email protected] Keywords: apoptosis, deacetylase, oxaliplatin, tumor-associated NADH oxidase (tNOX or ENOX2), sirtuin 1 (SIRT1) Received: October 20, 2016 Accepted: January 09, 2017 Published: January 21, 2017 ABSTRACT Oxaliplatin belongs to the platinum-based drug family and has shown promise in cancer treatment. The major mechanism of action of platinum compounds is to form platinum–DNA adducts, leading to DNA damage and apoptosis. Accumulating evidence suggests that they might also target non-DNA molecules for their apoptotic activity. We explored the effects of oxaliplatin on a tumor-associated NADH oxidase (tNOX) in gastric cancer lines. In AGS cells, we found that the oxaliplatin-inhibited tNOX effectively attenuated the NAD+/NADH ratio and reduced the deacetylase activity of an NAD+-dependent sirtuin 1, thereby enhancing p53 acetylation and apoptosis. Similar results were also observed in tNOX-knockdown AGS cells. -

Mouse Anti-Human COX17 Monoclonal Antibody, Clone 5H3 (CABT-B10023) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Mouse anti-Human COX17 monoclonal antibody, clone 5H3 (CABT-B10023) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Immunogen COX17 (NP_005685,2a.a. ~ 63 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. Isotype IgG2b Source/Host Mouse Species Reactivity Human Clone 5H3 Conjugate Unconjugated Applications WB, IHC, sELISA, ELISA Sequence Similarities MPGLVDSNPAPPESQEKKPLKPCCACPETKKARDACIIEKGEEHCGHLIEAHKECMRALGFKI Format Liquid Buffer In 1x PBS, pH 7.2 Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. BACKGROUND Introduction Cytochrome c oxidase (COX), the terminal component of the mitochondrial respiratory chain, catalyzes the electron transfer from reduced cytochrome c to oxygen. This component is a heteromeric complex consisting of 3 catalytic subunits encoded by mitochondrial genes and multiple structural subunits encoded by nuclear genes. The mitochondrially-encoded subunits function in electron transfer, and the nuclear-encoded subunits may function in the regulation and assembly of the complex. This nuclear gene encodes a protein which is not a structural subunit, but may be involved in the recruitment of copper to mitochondria for incorporation into the COX apoenzyme. This protein shares 92% amino acid sequence identity with mouse and rat Cox17 proteins. This gene is no longer considered to be a candidate gene for COX deficiency. A pseudogene COX17P has been found on chromosome 13. [provided by RefSeq, Jul 2008] 45-1 Ramsey -

Genome-Wide Identification of Mrnas Associated with the Protein SMN Whose Depletion Decreases Their Axonal Localization
Downloaded from rnajournal.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Genome-wide identification of mRNAs associated with the protein SMN whose depletion decreases their axonal localization FLORENCE RAGE,1,2,3 NAWAL BOULISFANE,1,2,3 KHALIL RIHAN,1,2,3 HENRY NEEL,1,2,3,4 THIERRY GOSTAN,1,2,3 EDOUARD BERTRAND,1,2,3 RÉMY BORDONNÉ,1,2,3 and JOHANN SORET1,2,3,5 1Institut de Génétique Moléculaire de Montpellier UMR 5535, 34293 Montpellier Cedex 5, France 2Université Montpellier 2, 34095 Montpellier Cedex 5, France 3Université Montpellier 1, 34967 Montpellier Cedex 2, France ABSTRACT Spinal muscular atrophy is a neuromuscular disease resulting from mutations in the SMN1 gene, which encodes the survival motor neuron (SMN) protein. SMN is part of a large complex that is essential for the biogenesis of spliceosomal small nuclear RNPs. SMN also colocalizes with mRNAs in granules that are actively transported in neuronal processes, supporting the hypothesis that SMN is involved in axonal trafficking of mRNPs. Here, we have performed a genome-wide analysis of RNAs present in complexes containing the SMN protein and identified more than 200 mRNAs associated with SMN in differentiated NSC-34 motor neuron-like cells. Remarkably, ∼30% are described to localize in axons of different neuron types. In situ hybridization and immuno-fluorescence experiments performed on several candidates indicate that these mRNAs colocalize with the SMN protein in neurites and axons of differentiated NSC-34 cells. Moreover, they localize in cell processes in an SMN-dependent manner. Thus, low SMN levels might result in localization deficiencies of mRNAs required for axonogenesis.