Nuclear and Cytoplasmic Genetic Variation in Picea: DNA Markers for Evaluating Past Migration, Introgression and Evolutionary History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Chrysomyxa Rust Species on the Endemic Plant, Picea Asperata in Western China, and Expanded Description of C
Phytotaxa 292 (3): 218–230 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.292.3.2 Two new Chrysomyxa rust species on the endemic plant, Picea asperata in western China, and expanded description of C. succinea JING CAO1, CHENG-MING TIAN1, YING-MEI LIANG2 & CHONG-JUAN YOU1* 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing 100083, China 2Museum of Beijing Forestry University, Beijing 100083, China *Corresponding author: [email protected] Abstract Two new rust species, Chrysomyxa diebuensis and C. zhuoniensis, on Picea asperata are recognized by morphological characters and DNA sequence data. A detailed description, illustrations, and discussion concerning morphologically similar and phylogenetically closely related species are provided for each species. From light and scanning electron microscopy observations C. diebuensis is characterized by the nailhead to peltate aeciospores, with separated stilt-like base. C. zhuoni- ensis differs from other known Chrysomyxa species in the annulate aeciospores with distinct longitudinal smooth cap at ends of spores, as well as with a broken, fissured edge. Analysis based on internal transcribed spacer region (ITS) partial gene sequences reveals that the two species cluster as a highly supported group in the phylogenetic trees. Correlations between the morphological and phylogenetic features are discussed. Illustrations and a detailed description are also provided for the aecia of C. succinea in China for the first time. Keywords: aeciospores, molecular phylogeny, spruce needle rust, taxonomy Introduction Picea asperata Mast.is native to western China, widely distributed in Qinghai, Gansu, Shaanxi and western Sichuan. -

Population Genomic Analysis Reveals That Homoploid Hybrid Speciation Can Speciation Hybrid Homoploid Reveals That Analysis Genomic Population E Origin
PROF. YONGSHUAI SUN (Orcid ID : 0000-0002-6926-8406) PROF. JIANQUAN LIU (Orcid ID : 0000-0002-4237-7418) Population genomic analysis reveals that homoploid hybrid speciation can be a lengthy process Dafu Ru1 *, Yongshuai Sun1,2*, Donglei Wang1*, Yang Chen1, Tianjing Wang1, Quanjun Hu1, Richard J. Abbott3, Jianquan Liu1# 1Key Laboratory for Bio-resource and Eco-environment of Ministry of Education, College of Life Sciences, Sichuan University, Chengdu 610065, P. R. China 2CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, P. R. China 3School of Biology, Mitchell Building, University of St Andrews, St Andrews, Fife KY16 9TH, UK Article *equal contributions to this work; #corresponding author ([email protected]) Abstract An increasing number of species are thought to have originated by homoploid hybrid speciation (HHS), but in only a handful of cases are details of the process known. A previous study indicated that Picea purpurea, a conifer in the Qinghai-Tibet Plateau (QTP), originated through HHS from P. likiangensis and P. wilsonii. To investigate this origin in more detail we analyzed transcriptome data for 114 individuals collected from 34 populations of the three Picea species from their core distributions in the QTP. Phylogenetic, principal component and admixture analyses of nuclear SNPs showed the species to be delimited genetically and that P. purpurea was admixed with approximately 60% of its ancestry derived from P. wilsonii and 40% from P. likiangensis. Coalescent simulations revealed the best-fitting model of origin involved formation of an intermediate hybrid lineage between P. likiangensis and P. -

Genetic Diversity and Conservation of Picea Chihuahuana Martínez: a Review
Vol. 13(28), pp. 2786-2795, 9 July, 2014 DOI: 10.5897/AJB2014.13645 Article Number: CADB48845877 ISSN 1684-5315 African Journal of Biotechnology Copyright © 2014 Author(s) retain the copyright of this article http://www.academicjournals.org/AJB Review Genetic diversity and conservation of Picea chihuahuana Martínez: A review Quiñones-Pérez, Carmen Zulema1, Sáenz-Romero, Cuauhtémoc2 and Wehenkel, Christian1* 1Institute of Forestry and Wood Industry, Universidad Juárez del Estado de Durango, Durango, México. 2Institute of Agricultural and Forestry Research, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, México. Received 20 January, 2014; Accepted 16 June, 2014 The conservation of genetic diversity in tree populations is an essential component of sustainable forest management. Picea chihuahuana Martínez is an endemic conifer species in Mexico and is considered to be endangered. P. chihuahuana covers a total area of no more than 300 ha at the Sierra Madre Occidental, a mountain range that harbor a high diversity of tree species. There are 40 populations of the species that have been identified in the region, and it cannot be found elsewhere. These populations form clusters within gallery forests and are usually associated with eight other tree genera. The P. chihuahuana community is mostly well preserved. Owing to its remarkable characteristics and high conservation value, P. chihuahuana has been the subject of several studies aimed at learning more about the genetic structure, ecology and potential effects of climate change. However, the overall applicability of such studies is to confirm a dataset to develop management tools to help decision makers and to implement preservation and conservation strategies using genetic diversity. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0263516 A1 CYR (43) Pub
US 20090263516A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0263516 A1 CYR (43) Pub. Date: Oct. 22, 2009 (54) PLANT EXTRACT COMPOSITION AND Publication Classification THEIR USE TO MODULATE CELLULAR (51) Int. Cl. ACTIVITY A636/8962 (2006.01) A636/00 (2006.01) (75) Inventor: Benoit CYR, St. Augustin de A6IP35/00 (2006.01) Desmaures (CA) CI2N 5/06 (2006.01) Correspondence Address: A6IR 36/3 (2006.01) SHEPPARD, MULLIN, RICHTER & HAMPTON A 6LX 36/899 (2006.01) LLP (52) U.S. Cl. ......... 424/754; 424/725; 435/375; 424/774; 990 Marsh Road 424/779; 424/755; 424/750; 424/777 Menlo Park, CA 94025 (US) (57) ABSTRACT (73) Assignee: Biopharmacopae Design Extracts from plant material, or semi-purified/purified mol International Inc., Saint-Foy (CA) ecules or compounds prepared from the extracts that demon strate the ability to modulate one or more cellular activities (21) Appl. No.: 12/263,114 are provided. The extracts are capable of slowing down, inhibiting or preventing cell migration, for example, the (22) Filed: Oct. 31, 2008 migration of endothelial cells or neoplastic cells and thus, the use of the extracts to slow down, inhibit or prevent abnormal Related U.S. Application Data cell migration in an animal is also provided. Methods of selecting and preparing the plant extracts and methods of (63) Continuation of application No. 10/526,387, filed on screening the extracts to determine their ability to modulate Oct. 6, 2005, now abandoned, filed as application No. one or more cellular activity are described. The purification or PCT/CA03/01284 on Sep. -

Picea Schrenkiana Tree-Ring Chronologies Development and Vegetation Index Reconstruction for the Alatau Mountains, Central Asia
GEOCHRONOMETRIA 45 (2018): 107–118 DOI 10.1515/geochr-2015-0091 Available online at http://www.degruyter.com/view/j/geochr PICEA SCHRENKIANA TREE-RING CHRONOLOGIES DEVELOPMENT AND VEGETATION INDEX RECONSTRUCTION FOR THE ALATAU MOUNTAINS, CENTRAL ASIA TONGWEN ZHANG1, 2, 3, RUIBO ZHANG1, 2, 3, BO LU4, BULKAJYR T. MAMBETOV5, NURZHAN KELGENBAYEV5, DANIYAR DOSMANBETOV5, BAGILA MAISUPOVA5, FENG CHEN1, 2, 3, SHULONG YU1, 2, 3, HUAMING SHANG1, 2, 3 and LIPING HUANG6 1Institute of Desert Meteorology, China Meteorological Administration, Urumqi 830002, China 2Key Laboratory of Tree-ring Physical and Chemical Research of China Meteorological Administration, Urumqi 830002, China 3Key Laboratory of Tree-ring Ecology of Uigur Autonomous Region, Urumqi 830002, China 4Laboratory for Climate Studies, National Climate Center, China Meteorological Administration, Beijing 100081 China 5Almaty Branch of Kazakh Scientific Research Institute of Forestry, Ministries of Agriculture, Almaty, 050010 Kazakhstan 6Institute of Modern Forestry, Xinjiang Academy of Forestry Science, Urumqi 830000, China Received 8 January 2018 Accepted 16 April 2018 Abstract: In this study, a total of 176 tree cores from Schrenk spruce (Picea schrenkiana) were used to establish a tree-ring chronology and a 167-year July–October normalized differential vegetation in- dex (NDVI) for the Alatau Mountains in Central Asia was reconstructed using this newly developed chronology. The tree-ring based NDVI reconstruction tracks the observed data well (r=0.577, p<0.01, n=25) and precisely captures the drought events recorded in historical documents that occurred over a large area in 1917 and 1938. After applying a 21-year moving average, three dense (1860–1870, 1891–1907, and 1950–1974) and three sparse (1871–1890, 1908–1949, and 1975–2006) vegetation coverage periods were found in this reconstruction. -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Introgressive Hybridization and Phylogenetic Relationships Between Norway, Picea Abies (L.) Karst., and Siberian, P
Heredity 74 (1995) 464—480 Received 10 December 1993 Genetical Society of Great Britain Introgressive hybridization and phylogenetic relationships between Norway, Picea abies (L.) Karst., and Siberian, P. obovata Ledeb., spruce species studied by isozyme loci KONSTANTIN V. KRUTOVSKII*t & FRITZ BERGMANNt 1-Laboratory of Population Genetics, N. I. Vavilov Institute of General Genetics, Russian Academy of Sciences, GSP- 1 Moscow 1178098-333, Russia and 1-Department of Forest Genetics and Forest Tree Breeding, Georg-August University of GOttingen, Büsgenweg 2, 37077 Gottingen, Germany Weanalysed patterns of genetic variation at 26 isozyme loci across the area of two main forest- forming spruce species in Eurasia, Norway spruce (Picea abies (L.) Karst.) and Siberian spruce (P. obovata Ledeb.). Ten seed samples from distant parts of the P. abies—P. obovata area and from a supposedly wide zone of introgressive hybridization between them were investigated. A very high level of allozyme variation was found in populations of both species. As parameters of gene diversity, the mean number of alleles per locus, percentage of polymorphic loci (95 per cent criterion) and expected heterozygosity averaged 2.8, 61.5 and 0.252 for P. abies and 2.4, 61.5 and 0.213 for P. obovata, respectively. Norway and Siberian spruces turned out to be extremely similar genetically. We did not find any fixed allele differences between them, i.e. there were no diagnostic loci and only a few alleles could be characteristic of some populations. Cluster and multivariate analyses have shown that these two species should be considered as two closely related subspecies or two geographical races of one spruce species undergoing considerable gene exchange. -

Wild Apple Growth and Climate Change in Southeast Kazakhstan
Article Wild Apple Growth and Climate Change in Southeast Kazakhstan Irina P. Panyushkina 1,* ID , Nurjan S. Mukhamadiev 2, Ann M. Lynch 1,3, Nursagim A. Ashikbaev 2, Alexis H. Arizpe 1, Christopher D. O’Connor 4, Danyar Abjanbaev 2, Gulnaz Z. Mengdbayeva 2 and Abay O. Sagitov 2 1 Laboratory of Tree-Ring Research, University of Arizona, 1215 W. Lowell St., Tucson, AZ 85721, USA; [email protected] (A.M.L.); [email protected] (A.H.A.) 2 Z.H. Zhiembaev Research Institute of Plant Protections and Quarantine, Almaty 050070, Kazakhstan; [email protected] (N.S.M.); [email protected] (N.A.A.); [email protected] (D.A.); [email protected] (G.Z.M.); [email protected] (A.O.S.) 3 U.S. Forest Service, Rocky Mountain Research Station, Tucson, AZ 85721, USA; [email protected] 4 U.S. Forest Service, Rocky Mountain Research Station, Missoula, MT 59801, USA; [email protected] * Correspondence: [email protected] Received: 31 August 2017; Accepted: 22 October 2017; Published: 26 October 2017 Abstract: Wild populations of Malus sieversii [Ldb.] M. Roem are valued genetic and watershed resources in Inner Eurasia. These populations are located in a region that has experienced rapid and on-going climatic change over the past several decades. We assess relationships between climate variables and wild apple radial growth with dendroclimatological techniques to understand the potential of a changing climate to influence apple radial growth. Ring-width chronologies spanning 48 to 129 years were developed from 12 plots in the Trans-Ili Alatau and Jungar Alatau ranges of Tian Shan Mountains, southeastern Kazakhstan. -

Autotoxicity and Allelopathy of 3,4-Dihydroxyacetophenone Isolated from Picea Schrenkiana Needles
Molecules 2011, 16, 8874-8893; doi:10.3390/molecules16108874 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Autotoxicity and Allelopathy of 3,4-Dihydroxyacetophenone Isolated from Picea schrenkiana Needles Xiao Ruan 1,2, Zhao-Hui Li 2, Qiang Wang 2,4,*, Cun-De Pan 3, De-An Jiang 1,* and G. Geoff Wang 4 1 College of Life Science, Zhejiang University, Hangzhou, 310058, China 2 Ningbo Institute of Technology, Zhejiang University, Ningbo, 315100, China 3 College of Forestry, Xinjiang Agricultural University, Urumqi, 830052, China 4 Department of Forestry and Natural Resources, Clemson University, 261 Lehotsky Hall, Clemson, SC 29634, USA * Authors to whom correspondence should be addressed; E-Mails: [email protected] (Q.W.); [email protected] (D.-A.J.); Tel.: +86-574-88134338 (Q.W.); +86-571-88206461 (D.-A.J.); Fax: +86-574-88229545(Q.W.). Received: 19 September 2011; in revised form: 9 October 2011 / Accepted: 17 October 2011 / Published: 24 October 2011 Abstract: Bioassay-guided fractionation of the diethyl ether fraction of a water extract of Picea schrenkiana needles led to the isolation of the phenolic compound 3,4-dihydroxy- acetophenone (DHAP). The allelopathic effects of DHAP were evaluated under laboratory conditions on P. schrenkiana, rice (Oryza sativa L.), wheat (Triticum aestivum L.), radish (Raphanus sativus L.), lettuce (Latuca sativa L.), cucumber (Cucumis sativus L.) and mung bean (Phaseolus radiatus L.). DHAP significantly inhibited seed germination and seedling growth of P. schrenkiana at concentrations of 2.5 mM and 0.5 mM (p < 0.05). Soil analysis revealed that P. schrenkiana forest soils contained exceptionally high DHAP concentrations (mean = 0.51 ± 0.03 mg/g dry soil), sufficient to inhibit natural P. -

Srovnání Morfologie Příčných Řezů Jehlic Smrků (Rod Picea)
Přírodovědecká fakulta v Olomouci Katedra Botaniky BAKALÁŘSKÁ PRÁCE Markéta Frdlíková Srovnání morfologie příčných řezů jehlic smrků (rod Picea) Vedoucí práce: RNDr. Radim J. Vašut, Ph.D. Obor: Biologie – geologie a ochrana ţivotního prostředí Místo a datum odevzdání: Olomouc, 26. července 2013 PROHLÁŠENÍ Prohlašuji, ţe jsem bakalářskou práci vykonávala samostatně, řídila jsem se pokyny svého vedoucího práce a předepsanou literaturou. V Olomouci, 26. července 2013 …..……………………. Markéta Frdlíková PODĚKOVÁNÍ Chtěla bych poděkovat svému vedoucímu práce RNDr. Radimu Janu Vašutovi, Ph.D. za ochotu pomoci, dodání materiálu a jeho času strávených na konzultačních hodinách. Dále bych chtěla poděkovat RNDr. Dagmar Skálové, Ph.D. za názornou ukázku řezání příčných řezů jehlic a seznámení s prací na mikrofotografickém systému Olympus DP 70. BIBLIOGRAFICKÁ IDENTIFIKACE Jméno a příjmení: Markéta Frdlíková Název práce : Srovnání morfologie příčných řezů jehlic smrků (rod Picea) Typ práce: Bakalářská Pracoviště: Katedra botaniky Vedoucí práce: RNDr. Radim J. Vašut, Ph.D. Rok obhajoby práce: 2013 Abstrakt: Úkolem mé bakalářské práce je charakterizovat vybrané druhy rodu Picea (smrku). Hlavním cílem je srovnání morfologie příčných řezů jehlic nejčastěji pěstovaných druhů u nás napříč hlavními fylogenetickými skupinami. Morfologické znaky jsou hlavním identifikačním znakem pro rozlišení jednotlivých druhů smrku (Picea). Práce byla doplněna o přehled dalších morfologických makroznaků uváděných v dendrologické literatuře, informace o rozšíření a další biologické -

Gentianaceae) from Xinjiang, China
A peer-reviewed open-access journal PhytoKeys 130:Gentianella 59–73 (2019) macrosperma, a new species of Gentianella from Xinjiang, China 59 doi: 10.3897/phytokeys.130.35476 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Gentianella macrosperma, a new species of Gentianella (Gentianaceae) from Xinjiang, China Hai-Feng Cao1, Ji-Dong Ya2, Qiao-Rong Zhang2, Xiao-Jian Hu2, Zhi-Rong Zhang2, Xin-Hua Liu3, Yong-Cheng Zhang4, Ai-Ting Zhang5, Wen-Bin Yu6,7 1 Shanghai Museum of TCM, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, Chi- na 2 Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Lanhei Road 132, Heilongtan, Kunming, Yunnan, 650201, China 3 Ili Botanical Garden, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Xinyuan, Xinjiang, 835815, China 4 Forestry Bureau of Xinyuan County, Xinyuan, Xinjiang, 835800, China 5 Xinjiang Agricultural Broadcasting and Television School, Xinyuan, Xinjiang, 835800, China 6 Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, Yunnan, China 7 Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Mengla 666303, Yunnan, China Corresponding author: Ji-Dong Ya ([email protected]) Academic editor: Cai Jie | Received 15 April 2019 | Accepted 8 August 2019 | Published 29 August 2019 Citation: Cao H-F, Ya J-D, Zhang Q-R, Hu X-J, Zhang Z-R, Liu X-H, Zhang Y-C, Zhang A-T, Yu W-B (2019) Gentianella macrosperma, a new species of Gentianella (Gentianaceae) from Xinjiang, China. In: Cai J, Yu W-B, Zhang T, Li D-Z (Eds) Revealing of the plant diversity in China’s biodiversity hotspots. -
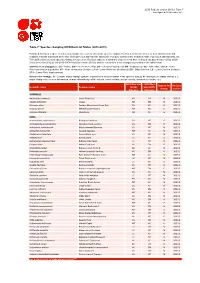
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying