Old and New Targets for Immunotherapy of B Cell Acute
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody–Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study
Ophthalmol Ther https://doi.org/10.1007/s40123-020-00280-8 ORIGINAL RESEARCH Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody–Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study Asim V. Farooq . Simona Degli Esposti . Rakesh Popat . Praneetha Thulasi . Sagar Lonial . Ajay K. Nooka . Andrzej Jakubowiak . Douglas Sborov . Brian E. Zaugg . Ashraf Z. Badros . Bennie H. Jeng . Natalie S. Callander . Joanna Opalinska . January Baron . Trisha Piontek . Julie Byrne . Ira Gupta . Kathryn Colby Received: April 21, 2020 Ó The Author(s) 2020, corrected publication 2020 ABSTRACT monomethyl auristatin F (MMAF), to myeloma cells. In the phase II DREAMM-2 study Introduction: Patients with relapsed or refrac- (NCT03525678), single-agent belamaf (2.5 mg/kg) tory multiple myeloma (RRMM) represent an demonstrated clinically meaningful anti-mye- unmet clinical need. Belantamab mafodotin loma activity (overall response rate 32%) in (belamaf; GSK2857916) is a first-in-class anti- patients with heavily pretreated disease. Micro- body–drug conjugate (ADC; or immunoconju- cyst-like epithelial changes (MECs) were com- gate) that delivers a cytotoxic payload, mon, consistent with reports from other MMAF- containing ADCs. Methods: Corneal examination findings from Digital Features To view digital features for this article patients in DREAMM-2 were reviewed, and the go to https://doi.org/10.6084/m9.figshare.12326546. clinical descriptions and accompanying images The original version of this article was revised based upon recent changes to the FDA label and guidance on D. Sborov the use of belamaf. Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA A. V. Farooq (&) Á A. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

(12) United States Patent (10) Patent No.: US 9,005,612 B2 Ledbetter Et Al
US009005612B2 (12) United States Patent (10) Patent No.: US 9,005,612 B2 Ledbetter et al. (45) Date of Patent: *Apr. 14, 2015 (54) BINDING DOMAIN-IMMUNOGLOBULIN 5,455,030 A 10/1995 Ladner et al. FUSION PROTEINS $599. A 3: BestInsley et al. al. (75) Inventors: Jeffrey A. Ledbetter, Seattle, WA (US); 5,530,101 A 6/1996 Queen et al. Martha S. Hayden-Ledbetter, Seattle, 5,580,756. A 12/1996 Linsley et al. WA (US) 5,595,7215,585,089 A 1431/1997 SAS,Kaminski et al.1 (73) Assignee: Emergent Product Development 5,597,707 A 1/1997 Marken et al. Seattle, LLC, Seattle, WA (US) 3.W - A 3.87 EaCee ea. (*) Notice: Subject to any disclaimer, the term of this 5,645,835 A 7/1997 Fell, Jr. et al. patent is extended or adjusted under 35 $22.9 A ck 8. 3. Earl 530,387.1 U.S.C. 154(b) by 0 days. 5,693,762.w A 12/1997 QueenOCC et Cal. al. ............ This patent is Subject to a terminal dis- 5,709,859 A 1/1998 Aruffo et al. claimer 5,714,147 A 2, 1998 Capon et al. 5,721, 108 A 2f1998 Robinson et al. (21) Appl. No.: 13/451,641 5,736,137 A 4, 1998 Anderson et al. 5,770,197 A 6/1998 Linsley et al. (22) Filed: Apr. 20, 2012 5,773.253 A 6/1998 Linsley et al. O O 5,776.456 A 7/1998 Anderson et al. (65) Prior Publication Data 5,795,572 A 8/1998 Diegel et al. -

Atpase, Na+/K+ Transporting, Alpha 3 Polypeptide Homologous to 3'UTR
HUGO ID Name Nalm-6 TOM-1 Reh Karpas-422 DoHH -2 SU-DHL-5 Namalwa DG-75 Ramos Raji BEL EHEB BONNA-12 L-428 DEL BCP-1 BC-3 BCBL-1 JSC-1 PEL-SY HBL-6 DS-1 RPMI-8226 NCI-H929 L-363 SK-MM-2 ATP1A3 ATPase, Na+/K+ transporting, alpha 3 polypeptide CD24 homologous to 3'UTR of human CD24 gene ABCC5 multidrug resistance-associated protein (MRP5) CD72 CD72 antigen TCL1A Tcell leukemia/lymphoma 1 ITGB2 Integrin, beta 2 (antigen CD18 (p95)) ? nuclear ribonucleoprotein particle (hnRNP) SGT1 suppressor of G2 allele of skp1 homolog DNMT 1 DNA (cytosine-5-)-methyltransferase 1 GALE UDP-Galactose 4 epimerase (GALE) HADHSC L-3-hydroxyacyl-CoA dehydrogenase LIG4 DNA ligase IV LIG1 Ligase I, DNA, ATP-dependent CEBPG CCAA T/enhancer binding protein (C/EBP), gamma DCK Deoxycytidine kinase TCEA1 TRANSCRIPTION ELONGATION FACTOR S-II TCN 1 TRANSCOBALAMIN I PRECURSOR POLA2 DNA polymerase alpha subunit CCNG2 cyclin G2 RNPC1 Finkel-Biskis-Reilly murine sarcoma virus; Human seb4D RNPC1 Finkel-Biskis-Reilly murine sarcoma virus; Human seb4D DGKD Diacylglycerol kinase delta KIAA0220 Polycystic kidney disease protein 1 KIAA0220 calcium-dependent group X phospholipase A2 KIAA0220 calcium-dependent group X phospholipase A2 ALDH5A1 NAD+-dependent succinate-semialdehyde dehydrogenase CCNG2 Polycystic kidney disease 1 (autosomal dominant) PDCD4 nuclear antigen H731-like protein SSH3BP1 eps8 binding protein e3B1 MAP4K2 B lymphocyte serine/threonine protein kinase (GC kinase) MAPRE2 novel T-cell activation protein ZNFN1A Ikaros/LyF-1 homolog (hIk-1) FLJ22624 clone 23799 KIAA0355 -

Πανελλήνιο Αιματολογικό Συνέδριο 28 Annual Meeting of the Hellenic Society of Haematology Η Η Βαλκανική Ημερίδα 128 Balkan Day
ΤΟΜΟΣ 8 - ΤΕΥΧΟΣ 3 ΟΚΤΩΒΡΙΟΣ - ΔΕΚΕΜΒΡΙΟΣ 2017 Αίμα 8 (3) Πρακτικά Εκπαιδευτικού Προγράμματος 28ου Πανελληνίου Αιματολογικού Συνεδρίου Αιματολογικού 28ου Πανελληνίου Προγράμματος Εκπαιδευτικού Πρακτικά ISSN: 1108-2682 Διευθυντής Σύνταξης: Καθηγητής Φώτης Ν. Μπερής Συνεκδότες: Καθηγήτρια Ελένη A. Παπαδάκη, Καθηγητής Κωνσταντίνος Τσαταλάς Aναπληρωτής Εκδότης: Επίκουρος Καθηγητής Θεόδωρος Π. Βασιλακόπουλος ΕΛΛΗΝΙΚΗ ΑΙΜΑΤΟΛΟΓΙΚΗ ΕΤΑΙΡΕΙΑ HELLENIC SOCIETY OF HAEMATOLOGY 0 Πανελλήνιο Αιματολογικό Συνέδριο 28 Annual meeting of the Hellenic Society of Haematology η η Βαλκανική Ημερίδα 128 Balkan Day 2-4 Nοεμβρίου 2017 Μέγαρο Διεθνές Συνεδριακό Κέντρο Αθηνών Νovember 2-4, 2017 Megaron, Athens International Conference Center ΟΚΤΩΒΡΙΟΣ - ΔΕΚΕΜΒΡΙΟΣ 2017 ΔΕΚΕΜΒΡΙΟΣ - ΟΚΤΩΒΡΙΟΣ Γραμματεία: Β. ΒΟΥΡΑΖΕΡΗΣ & ΣΙΑ O.Ε. Παπαδιαμαντοπούλου 4 & Βασ. Σοφίας, 115 28 Αθήνα, Τηλ.: 210 72 54 360, Fax: 210 72 54 363, e-mail: [email protected], website: www.vitacongress.gr ΠΡΑΚΤΙΚΑ ΕΚΠΑΙΔΕΥΤΙΚΟΥ ΠΡΟΓΡΑΜΜΑΤΟΣ 28ου Πανελληνίου Αιματολογικού Συνεδρίου HELLENIC SOCIETY OF HAEMATOLOGY ΕΛΛΗΝΙΚΗ ΑΙΜΑΤΟΛΟΓΙΚΗ ΕΤΑΙΡΕΙΑ The Journal Περιοδική Έκδοση της of the Hellenic Society of Ελληνικής ΑιμΑτολογικης HEMATOLOGY Εταιρείας HELLENIC SOCIETY OF HAEMATOLOGY ΕΛΛΗΝΙΚΗ ΑΙΜΑΤΟΛΟΓΙΚΗ ΕΤΑΙΡΕΙΑ BOARD OF THE HELLENIC ΔΙΟΙΚΗΤΙΚΟ ΣυΜΒΟυΛΙΟ SOCIETY OF HAEMATOLOGY ΤΗΣ ΕΛΛΗνΙΚΗΣ ΑΙΜΑΤΟΛΟΓΙΚΗΣ ΕΤΑΙΡΕΙΑΣ President: Panayiotis Panayiotidis Πρόεδρος: Παναγιώτης Παναγιωτίδης Vice Presidents: Elisavet Grouzi Αντιπρόεδροι: Ελισάβετ Γρουζή Evangelos Terpos Ευάγγελος Τέρπος Secretary General: -
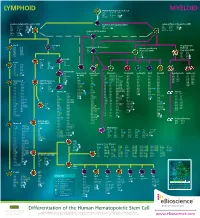
Lymphoid Myeloid
LYMPHOID Human Hematopoietic Stem Cell MYELOID CD34 CD117 (c-kit) CD338 CD38low/neg CD133 linneg CD59 CD135 (Flt3) GATA2 CD90 (Thy1) CD164 TdT Common Lymphoid Progenitor (CLP) Multi-Potent Progenitor (MPP) Common Myeloid Progenitor (CMP) low neg CD33 CD123low CD174 CD7 CD117 (c-kit) C/EBPα CD34 linneg neg CD34 CD131 Ikaros CD10 CD127 GATA2 CD135 (Flt3) TdT CD24neg CD135 (Flt3) GATA3 CD45RA CD164 PU.1 CD34 CD164 PU.1high Common DC Progenitor CD117 (c-kit) CD173 CD38 HLA-DR TdT CD45RA Aiolos CD11c dim CD90low c-mybhigh CD33 Pro-B Pre-NK/T Megakaryocyte- CD10 CD124 CD5 Conventional DC Precursor Erythroid CD19 CD164 CD34 CD11c Granulocyte-Myeloid CD20 CD252 CD14neg Progenitor (GMP) Progenitor (MEP) CD22 CD268 CD33dim CD33 CD131 CD34neg CD275 CD34 CD45RA CD34 CD164 CD72 HLA-DR CD36 FOG-1 CD74 CD123 PU.1 CD110 GATA1 CD123high GATA2 Pre-T Pro-NK CD7 Pre-B CD1a CD28 CD10neg CD2 CD34 neg CD10 CD124 CD34 CD3 CD45 CD117 (c-kit) CD19 CD164 CD5 CD127 (IL-7Rα) CD20 CD252 CD7high NOTCH1 CD22 CD268 CD34neg CD275 CD72 HLA-DR Immature NK CD74 IgM CD34neg Plasmacytoid Conventional Monocyte Neutrophil Eosinophil Mast Basophil Megakaryocyte Erythrocyte CD79a Pax5 CD94neg DC (pDC) DC (cDC) CD4low CD85a (ILT5) CD171 CD10 CD9 CD9 CD9 CD41 CD51 CD35 CD236 CD117 (c-kit) CD9 CD85d (ILT4) CD172a (SIRPα) CD15 CD11b CD11b CD11a CD42a CD61 CD44 CD236R CD4 CD1c CD122 CD11b CD85h (ILT1) CD172b CD16b CD15 CD15 CD11b CD42b CD110 CD123 CD238 CD11c neg CD1d CD161 CD11c CD85j (ILT2) CD180 CD17 CD24 CD24 CD13 CD42c CD112 CD173 CD239 CD45RA CD2 Ets-1 CD13 -

Human CD Marker Chart Reviewed by HLDA1 Bdbiosciences.Com/Cdmarkers
BD Biosciences Human CD Marker Chart Reviewed by HLDA1 bdbiosciences.com/cdmarkers 23-12399-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD93 C1QR1,C1qRP, MXRA4, C1qR(P), Dj737e23.1, GR11 – – – – – + + – – + – CD220 Insulin receptor (INSR), IR Insulin, IGF-2 + + + + + + + + + Insulin-like growth factor 1 receptor (IGF1R), IGF-1R, type I IGF receptor (IGF-IR), CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD94 KLRD1, Kp43 HLA class I, NKG2-A, p39 + – + – – – – – – CD221 Insulin-like growth factor 1 (IGF-I), IGF-II, Insulin JTK13 + + + + + + + + + CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD178, FASLG, APO-1, FAS, TNFRSF6, CD95L, APT1LG1, APT1, FAS1, FASTM, CD95 CD178 (Fas ligand) + + + + + – – IGF-II, TGF-b latency-associated peptide (LAP), Proliferin, Prorenin, Plasminogen, ALPS1A, TNFSF6, FASL Cation-independent mannose-6-phosphate receptor (M6P-R, CIM6PR, CIMPR, CI- CD1d R3G1, R3 b-2-Microglobulin, MHC II CD222 Leukemia