Identification of Candidate ATP-Binding Cassette Transporter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Wide Analysis of ATP-Binding
Tian et al. BMC Genomics (2017) 18:330 DOI 10.1186/s12864-017-3706-6 RESEARCH ARTICLE Open Access Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweetpotato whitefly, Bemisia tabaci Lixia Tian1, Tianxue Song2, Rongjun He1, Yang Zeng1, Wen Xie1, Qingjun Wu1, Shaoli Wang1, Xuguo Zhou3* and Youjun Zhang1* Abstract Background: ABC transporter superfamily is one of the largest and ubiquitous groups of proteins. Because of their role in detoxification, insect ABC transporters have gained more attention in recent years. In this study, we annotated ABC transporters from a newly sequenced sweetpotato whitefly genome. Bemisia tabaci Q biotype is an emerging global invasive species that has caused extensive damages to field crops as well as ornamental plants. Results: A total of 55 ABC transporters containing all eight described subfamilies (A to H) were identified in the B. tabaci Q genome, including 8 ABCAs, 3 ABCBs, 6 ABCCs, 2 ABCDs, 1 ABCE, 3 ABCFs, 23 ABCGs and 9 ABCHs. In comparison to other species, subfamilies G and H in both phloem- and blood-sucking arthropods are expanded. The temporal expression profiles of these 55 ABC transporters throughout B. tabaci developmental stages and their responses to imidacloprid, a neonicotinoid insecticide, were investigated using RNA-seq analysis. Furthermore, the mRNA expression of 24 ABC transporters (44% of the total) representing all eight subfamilies was confirmed by the quantitative real-time PCR (RT-qPCR). Furthermore, mRNA expression levels estimated by RT-qPCR and RNA-seq analyses were significantly correlated (r =0.684,p <0.01). Conclusions: It is the first genome-wide analysis of the entire repertoire of ABC transporters in B. -

Evolutionary and Functional Studies on Microsporidian ATP-Binding
Infection, Genetics and Evolution 68 (2019) 136–144 Contents lists available at ScienceDirect Infection, Genetics and Evolution journal homepage: www.elsevier.com/locate/meegid Evolutionary and functional studies on microsporidian ATP-binding T cassettes: Insights into the adaptation of microsporidia to obligated intracellular parasitism Qiang Hea, Charles R. Vossbrinckc, Qiong Yangd, Xian-Zhi Menga, Jian Luoa, Guo-Qing Pana, ⁎ ⁎ Ze-Yang Zhoua,b, , Tian Lia, a State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400715, China b College of Life Science, Chongqing Normal University, Chongqing 400047, China c Department of Soil and Water, The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, CT 06511, USA d Sericulture and Agri-Food Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China ARTICLE INFO ABSTRACT Keywords: ATP-binding cassette (ABC) transporters comprise the largest family of transmembrane proteins and are found in Microsporidian all domains of life. The ABCs are involved in a variety of biological processes and as exporters play important ABC transporter roles in multidrug resistance. However, the ABC transporters have not been addressed in microsporidia, which Genome are a very large group of obligate intracellular parasites that can infect nearly all animals, including humans. Evolution Here, a total of 234 ABC transporters were identified from 18 microsporidian genomes and classified intofive Nosema bombycis subfamilies, including 74 ABCBs, 2 ABCCs, 18 ABCEs, 15 ABCFs, 102 ABCGs and 23 uncategorized members. Two subfamilies, ABCA and ABCD, are found in most organisms, but lost in microsporidia. Phylogenetic analysis indicated that microsporidian ABCB and ABCG subfamilies expanded by recent gene duplications, which re- sulted in the two largest subfamilies in microsporidia. -

Drosophila Melanogaster”
| PRIMER More than Meets the Eye: A Primer for “Timing of Locomotor Recovery from Anoxia Modulated by the white Gene in Drosophila melanogaster” Bradley M. Hersh1 Department of Biology, Allegheny College, Meadville, Pennsylvania 16335 ORCID ID: 0000-0003-2098-4417 (B.M.H.) SUMMARY A single gene might have several functions within an organism, and so mutational loss of that gene has multiple effects across different physiological systems in the organism. Though the white gene in Drosophila melanogaster was identified originally for its effect on fly eye color, an article by Xiao and Robertson in the June 2016 issue of GENETICS describes a function for the white gene in the response of Drosophila to oxygen deprivation. This Primer article provides background information on the white gene, the phenomenon of pleiotropy, and the molecular and genetic approaches used in the study to demonstrate a new behavioral function for the white gene. KEYWORDS education; Drosophila; pleiotropy; behavior TABLE OF CONTENTS Abstract 1369 Molecular Nature of the white Gene 1370 The Challenge of Pleiotropy 1370 Tissue-Specific Expression and RNA Interference (RNAi) 1371 Understanding the Experimental Details 1372 Establishing a behavioral phenotype 1372 Introgression: eliminating the trivial 1372 Dosage and position effect: complicating the story 1373 Molecular tricks: dissecting function and location of action 1373 Suggestions for Classroom Use 1374 Questions for Discussion 1374 HE white gene was the first Drosophila melanogaster the first attached-X and ring-X chromosome variants), is re- Tmutant discovered by Thomas Hunt Morgan in 1910, ported to have exclaimed “Oh, I do hope the white-eyed flyis following an exhaustive search for variant forms of the fly still alive” from her hospital bed after having just delivered (Morgan 1910). -

Sex – Linkage and Autosexing in Waterfowl
SEX – LINKAGE AND AUTOSEXING IN WATERFOWL CONTENTS Page The principles of sex-linkage .. .. .. .. .. .. 1 Sex-linkage in the common duck .. .. .. .. .. 3 Sex-linkage in the Muscovy duck .. .. .. .. .. 11 Sex-linkage in the common goose .. .. .. .. .. 12 Sex-linkage in the Chinese goose .. .. .. .. .. 14 Sex-linkage in the Mute swan .. .. .. .. .. .. 14 Autosexing in waterfowl .. .. .. .. .. .. 15 The Z chromosome .. .. .. .. .. .. .. 17 Summary .. .. .. .. .. .. .. .. .. 18 References .. .. .. .. .. .. .. .. .. 18 ------------------------ August 1977 F.M. Lancaster, Original draft published in the Formerly of : B.W.A. Journal – Dec. 1977 National Inst. of Poultry Husbandry, and April 1978. Harper Adam Agricultural College, Updated: November, 2016 Newport, Shropshire (Now Harper Adams University) 1 SEX – LINKAGE AND AUTOSEXING IN WATERFOWL It is only fair to state that the need for early sex determination, through sex linked crosses in waterfowl, is much less than in other classes of poultry. This is because it is easier to vent sex the day-olds of these species with very little training. Moreover, crossbreeding is rarely an option for exhibition and ornamental breeders. The exception is in commercial table duckling production where unfortunately since only white breeds are used, sex-linkage cannot be exploited. There may be some, however, who feel unable to attempt vent sexing, particularly with goslings which are more difficult to manipulate and more vulnerable to rough handling. Others may be interested in sex-linked inheritance for its own sake regardless of any practical advantage. THE PRINCIPLES OF SEX – LINKAGE Without going into too much technical detail I would like to explain the principles underlying sex-linkage. For a more detailed account of these principles the reader is referred to the excellent bulletin by Chris Hann (1966). -

Polymorphisms of the Multidrug Pump ABCG2: a Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics
1521-009X/46/12/1886–1899$35.00 https://doi.org/10.1124/dmd.118.083030 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1886–1899, December 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Minireview Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics Niall Heyes, Parth Kapoor, and Ian D. Kerr School of Life Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom Received June 12, 2018; accepted September 20, 2018 Downloaded from ABSTRACT The widespread expression and polyspecificity of the multidrug to improve outcomes in cancer patients treated with tyrosine ABCG2 efflux transporter make it an important determinant of the kinase inhibitors, but the reasons for this are yet to be estab- pharmacokinetics of a variety of substrate drugs. Null ABCG2 lished, and this residue’s role in the mechanism of the protein is expression has been linked to the Junior blood group. Polymor- unexplored by current biochemical and structural approaches. phisms affecting the expression or function of ABCG2 may have Research into the less-common polymorphisms is confined to dmd.aspetjournals.org clinically important roles in drug disposition and efficacy. The in vitro studies, with several polymorphisms shown to decrease most well-studied single nucleotide polymorphism (SNP), Q141K resistance to anticancer agents such as SN-38 and mitoxantrone. (421C>A), is shown to decrease ABCG2 expression and activity, In this review, we present a systematic analysis of the effects of resulting in increased total drug exposure and decreased re- ABCG2 polymorphisms on ABCG2 function and drug pharmaco- sistance to various substrates. -

ABCG4: a Novel Human White Family ABC-Transporter Expressed in the Brain and Eye
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1591 (2002) 175–179 www.bba-direct.com Short sequence-paper ABCG4: a novel human white family ABC-transporter expressed in the brain and eye Susan Oldfield *, Christopher A. Lowry, Jon Ruddick, Stafford L. Lightman University Research Centre for Neuroendocrinology, BRI, University of Bristol, Marlborough Street, Bristol BS2 8HW, UK Received 24 July 2001; received in revised form 11 April 2002; accepted 24 May 2002 Abstract White family transporters are a group of ATP-binding cassette (ABC) proteins that show sequence homology to the Drosophila white gene product. The Drosophila protein is a subunit of heterodimeric transporters of precursors for eye-pigment synthesis. A novel, human member of this family (ABCG4) has been identified. Northern blotting shows that ABCG4 is expressed specifically in the brain and the eye. D 2002 Elsevier Science B.V. All rights reserved. Keywords: White gene; ABC-transporter; ABCG1; Sterol ATP-binding cassette (ABC) transporters form a large and its murine counterpart is known as ABCG1 or ABC8. superfamily of proteins that mediate the transport of a large Northern blotting has shown that the mRNAs encoding variety of diverse molecules across biological membranes in ABCG1 are present in several tissues, predominantly brain, an ATP-dependent manner [1]. The functional transporters spleen and lung [10]. contain two ATP-binding folds and two transmembrane The function of ABCG1 is unknown, although there is domains each with, usually, five or six membrane spanning evidence that suggests that it may have a role in cholesterol helices. -

Transcriptional and Post-Transcriptional Regulation of ATP-Binding Cassette Transporter Expression
Transcriptional and Post-transcriptional Regulation of ATP-binding Cassette Transporter Expression by Aparna Chhibber DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmaceutical Sciences and Pbarmacogenomies in the Copyright 2014 by Aparna Chhibber ii Acknowledgements First and foremost, I would like to thank my advisor, Dr. Deanna Kroetz. More than just a research advisor, Deanna has clearly made it a priority to guide her students to become better scientists, and I am grateful for the countless hours she has spent editing papers, developing presentations, discussing research, and so much more. I would not have made it this far without her support and guidance. My thesis committee has provided valuable advice through the years. Dr. Nadav Ahituv in particular has been a source of support from my first year in the graduate program as my academic advisor, qualifying exam committee chair, and finally thesis committee member. Dr. Kathy Giacomini graciously stepped in as a member of my thesis committee in my 3rd year, and Dr. Steven Brenner provided valuable input as thesis committee member in my 2nd year. My labmates over the past five years have been incredible colleagues and friends. Dr. Svetlana Markova first welcomed me into the lab and taught me numerous laboratory techniques, and has always been willing to act as a sounding board. Michael Martin has been my partner-in-crime in the lab from the beginning, and has made my days in lab fly by. Dr. Yingmei Lui has made the lab run smoothly, and has always been willing to jump in to help me at a moment’s notice. -
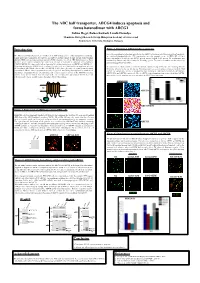
The ABC Half Transporter, ABCG4 Induces Apoptosis and Forms Heterodimer with ABCG1
The ABC half transporter, ABCG4 induces apoptosis and forms heterodimer with ABCG1 Zoltán Hegyi, Balázs Sarkadi, László Homolya Membrane Biology Research Group, Hungarian Academy of Sciences and Semmelweis University, Budapest, Hungary Introduction Figure 3. Functional ABCG4 induces apoptosis Since the morphological alterations observed in the ABCG4-expressing cells were indicative of apoptosis, The ABCG1 and ABCG4 proteins are members of the ATP binding cassette (ABC) transporter G subfamily. we examined phosphatidyl-serine (PS) translocation, an early apoptotic event in HEK293H cell lines Unlike most ABC transporters, the ABCG1 and ABCG4 proteins consist of only one nucleotide binding transiently transfected with the wt ABCG4 and its inactive mutant (KM) variant. PS translocation was domain (NBD) and one transmembrane domain (TMD), therefore are called ABC half-transporters. Some monitored by fluorescently labeled Annexin V binding (green). The total cell number was determined by members of the ABCG subfamily have proven to function as homodimers (ABCG2) or heterodimers nuclear staining by Hoechst (blue). (ABCG5/ABCG8). Previous results indicated potential heterodimerization between ABCG1 and ABCG4 (1). Regarding their function, ABCG1 has been proposed to play a role in cellular lipid/sterol regulation, whereas We found that the cultures transfected with wt ABCG4 contained a large number of cells exhibiting Annexin the function of ABCG4, the closest relative of ABCG1, is still elusive. Recently, we reported that functional V binding, whereas hardly any labeling for PS translocation was seen in cultures transfected with the KM expression of ABCG1 induces apoptosis in several cell types (2). Our finding was supported by rounded cell mutant. For comparison, Annexin V binding was also examined in cells transfected with the wt ABCG1, morphology, phosphatidyl-serine externalization, and elevated caspase 3 activity in the ABCG1-expressing ABCG1-KM, and ABCG2, respectively. -

Timeline of Genomics (1901–1950)*
Research Resource Timeline of Genomics (1901{1950)* Year Event and Theoretical Implication/Extension Reference 1901 Hugo de Vries adopts the term MUTATION to de Vries, H. 1901. Die Mutationstheorie. describe sudden, spontaneous, drastic alterations in Veit, Leipzig, Germany. the hereditary material of Oenothera. Thomas Harrison Montgomery studies sper- 1. Montgomery, T.H. 1898. The spermato- matogenesis in various species of Hemiptera and ¯nds genesis in Pentatoma up to the formation that maternal chromosomes only pair with paternal of the spermatid. Zool. Jahrb. 12: 1-88. chromosomes during meiosis. 2. Montgomery, T.H. 1901. A study of the chromosomes of the germ cells of the Metazoa. Trans. Am. Phil. Soc. 20: 154-236. Clarence Ervin McClung postulates that the so- McClung, C.E. 1901. Notes on the acces- called accessory chromosome (now known as the \X" sory chromosome. Anat. Anz. 20: 220- chromosome) is male determining. 226. Hermann Emil Fischer(1902 Nobel Prize Laure- 1. Fischer, E. and Fourneau, E. 1901. UberÄ ate for Chemistry) and Ernest Fourneau report einige Derivate des Glykocolls. Ber. the synthesis of the ¯rst dipeptide, glycylglycine. In Dtsch. Chem. Ges. 34: 2868-2877. 1902 Fischer introduces the term PEPTIDES. 2. Fischer, E. 1907. Syntheses of polypep- tides. XVII. Ber. Dtsch. Chem. Ges. 40: 1754-1767. 1902 Theodor Boveri and Walter Stanborough Sut- 1. Boveri, T. 1902. UberÄ mehrpolige Mi- ton found the chromosome theory of heredity inde- tosen als Mittel zur Analyse des Zellkerns. pendently. Verh. Phys -med. Ges. WÄurzberg NF 35: 67-90. 2. Boveri, T. 1903. UberÄ die Konstitution der chromatischen Kernsubstanz. Verh. Zool. -

Thomas Hunt Morgan
NATIONAL ACADEMY OF SCIENCES T HOMAS HUNT M ORGAN 1866—1945 A Biographical Memoir by A. H . S TURTEVANT Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1959 NATIONAL ACADEMY OF SCIENCES WASHINGTON D.C. THOMAS HUNT MORGAN September 25, 1866-December 4, 1945 BY A. H. STURTEVANT HOMAS HUNT MORGAN was born September 25, 1866, at Lexing- Tton, Kentucky, the son of Charlton Hunt Morgan and Ellen Key (Howard) Morgan. In 1636 the two brothers James Morgan and Miles Morgan came to Boston from Wales. Thomas Hunt Morgan's line derives from James; from Miles descended J. Pierpont Morgan. While the rela- tionship here is remote, geneticists will recognize that a common Y chromosome is indicated. The family lived in New England^ mostly in Connecticut—until about 1800, when Gideon Morgan moved to Tennessee. His son, Luther, later settled at Huntsville, Alabama. This Luther Morgan was the grandfather of Charlton Hunt Morgan; the latter's mother (Thomas Hunt Morgan's grand- mother) was Henrietta Hunt, of Lexington, whose father, John Wesley Hunt, came from Trenton, New Jersey, and was one of the early settlers at Lexington, where he became a hemp manufacturer. Ellen Key Howard was from an old aristocratic family of Baltimore, Maryland. Her two grandfathers were John Eager Howard (Colonel in the Revolutionary Army, Governor of Maryland from 1788 to 1791) and Francis Scott Key (author of "The Star-spangled Ban- ner"). Thomas Hunt Morgan's parents were related, apparently as third cousins. -

Y Chromosomes of the Proposed
Sequencing and Annotating New Mammalian Y Chromosomes A White Paper Proposal, July, 2006 Steve Rozen1, Wesley C. Warren2, George Weinstock3, Stephen J. O’Brien4, Richard A. Gibbs3, Richard K. Wilson2, David C. Page1 1 Howard Hughes Medical Institute, Whitehead Institute, and Department of Biology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02142 2 Genome Sequencing Center, Washington University School of Medicine, St Louis, Missouri 63108 3 Human Genome Sequencing Center, Baylor College of Medicine, Houston, Texas 77030 4 Laboratory of Genomic Diversity, National Cancer Institute, Frederick, Maryland 21702 Overview: Y chromosome sequence is essential for understanding human and mammalian evolution and biology The only Y chromosome that has been completely sequenced in any species is that of humans1,2, although sequencing of the chimpanzee and mouse Y chromosomes is in progress2-4. Here we propose to sequence and annotate the Y chromosomes of seven additional mammals at varying distances from human: Macaca mulatta (rhesus macaque) Callithrix jacchus (white-tufted-ear marmoset), Rattus norvegicus (laboratory rat), Bos taurus (domestic bull), Felis catus (domestic cat), Canis familiaris (dog), and Monodelphis domestica (laboratory opossum). We recognize that NHGRI approved rat Y chromosome sequencing as part of additional rat finishing5 and that the bull Y chromosome falls within the scope of the Bovine Genome Project, led by the Baylor sequencing center6. Inclusion of the rat and bull Y chromosomes is intended (i) to clarify collaborative intentions among the submitting sequencing centers (ii) to ensure coordination and agreement of the definitions of the most useful levels of completion of these chromosomes and (iii) to emphasize coherent biological rationales for sequencing and annotating the seven proposed Y chromosomes in terms of their relevance to human evolution, biology, and disease. -

Cholesterol Loading Suppresses the Atheroinflammatory Gene
www.nature.com/scientificreports OPEN Cholesterol loading suppresses the atheroinfammatory gene polarization of human macrophages induced by colony stimulating factors Jani Lappalainen1, Nicolas Yeung1, Su D. Nguyen1, Matti Jauhiainen2, Petri T. Kovanen1* & Miriam Lee‑Rueckert1 In atherosclerotic lesions, blood‑derived monocytes diferentiate into distinct macrophage subpopulations, and further into cholesterol‑flled foam cells under a complex milieu of cytokines, which also contains macrophage‑colony stimulating factor (M‑CSF) and granulocyte–macrophage‑ colony stimulating factor (GM‑CSF). Here we generated human macrophages in the presence of either M‑CSF or GM‑CSF to obtain M‑MØ and GM‑MØ, respectively. The macrophages were converted into cholesterol‑loaded foam cells by incubating them with acetyl‑LDL, and their atheroinfammatory gene expression profles were then assessed. Compared with GM‑MØ, the M‑MØ expressed higher levels of CD36, SRA1, and ACAT1, and also exhibited a greater ability to take up acetyl‑LDL, esterify cholesterol, and become converted to foam cells. M‑MØ foam cells expressed higher levels of ABCA1 and ABCG1, and, correspondingly, exhibited higher rates of cholesterol efux to apoA‑I and HDL2. Cholesterol loading of M‑MØ strongly suppressed the high baseline expression of CCL2, whereas in GM‑MØ the low baseline expression CCL2 remained unchanged during cholesterol loading. The expression of TNFA, IL1B, and CXCL8 were reduced in LPS‑activated macrophage foam cells of either subtype. In summary, cholesterol loading converged the CSF‑dependent expression of key genes related to intracellular cholesterol balance and infammation. These fndings suggest that transformation of CSF‑polarized macrophages into foam cells may reduce their atheroinfammatory potential in atherogenesis.