ANNUAL REPORT 2014 REPORT ANNUAL Ltd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2013 Technology Map of the European Strategic Energy Technology Plan
JRC SCIENCE AND POLICY REPORTS 2013 Technology Map of the European Strategic Energy Technology Plan Technology Descriptions Report EUR 26345 EN Joint Research Centre JRC13_TMAP_08AP14_P5-NS-Print.indd 1 09/04/2014 16:20:55 EUROPEAN COMMISSION Joint Research Centre Institute for Energy and Transport Contact: Johan Carlsson Address: Joint Research Centre, 3 Westerduinweg 1755 LE Petten the Netherlands E-mail: [email protected] Tel.: +31 224565341 Fax: +31 224565616 http://iet.jrc.ec.europa.eu/ http://www.jrc.ec.europa.eu/ This publication is a Scientific and Policy Report by the Joint Research Centre of the European Commission. LEGAL NOTICE Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC86357 EUR 26345 EN ISBN 978-92-79-34721-4 (print) ISBN 978-92-79-34720-7 (pdf) ISSN 1018-5593 (print) ISSN 1831-9424 (online) doi: 10.2790/9986 (print) doi: 10.2790/99812 (online) Luxembourg: Publications Office of the European Union, 2014 © European Union, 2014 Reproduction is authorised provided the source is acknowledged. Printed in Luxembourg JRC13_TMAP_08AP14_P5-NS-Print.indd 2 09/04/2014 16:20:55 2013 Technology Map of the European Strategic Energy Technology Plan (SET-Plan) Technology Descriptions JRC13_TMAP_08AP14_P5-NS-Print.indd 1 09/04/2014 16:20:55 JRC13_TMAP_08AP14_P5-NS-Print.indd 2 09/04/2014 16:20:56 TABLE OF CONTENTS 1. -

ADVISORY BOARDS Each Issue of Herbaigram Is Peer Reviewed by Various Members of Our Advisory Boards Prior to Publication
ADVISORY BOARDS Each issue of HerbaiGram is peer reviewed by various members of our Advisory Boards prior to publication . American Botanical Council Herb Research Dennis V. C. Awang, Ph.D., F.C.I.C., MediPiont Natural Gail B. Mahadr, Ph.D., Research Assistant Professor, Products Consul~ng Services, Ottowa, Ontario, Conodo Deportment o Medical Chemistry &Pharmacognosy, College of Foundation Pharmacy, University of Illinois, Chicago, Illinois Manuel F. Balandrin, R.Ph., Ph.D., Research Scien~st, NPS Rob McCaleb, President Pharmaceuticals, Salt LakeCity , Utah Robin J. Maries, Ph.D., Associate Professor of Botany, Brandon University, Brandon, Manitoba, Conodo Mi(hael J. Balidt, Ph.D., Director of the lns~tute of Econom ic Glenn Appelt, Ph.D., R.Ph., Author and Profess or Botany, the New York Botanical Gorden, Bronx, New York Dennis J. M(Kenna, Ph.D., Consulting Ethnophormocologist, Emeritus, University of Colorado, and with Boulder Beach Joseph M. Betz, Ph.D., Research Chemist, Center for Food Minneapolis, Minnesota Consulting Group Safety and Applied Nutri~on, Division of Natural Products, Food Daniel E. Moerman, Ph.D., William E. Stirton Professor of John A. Beutler, Ph.D., Natural Products Chemist, and Drug Administro~on , Washington, D.C. Anthropology, University of Michigon/ Deorbom, Dearborn, Michigan Notional Cancer Institute Donald J. Brown, N.D., Director, Natural Products Research Consultants; Faculty, Bastyr University, Seattle, Washington Samuel W. Page, Ph.D., Director, Division of Natural Products, Robert A. Bye, Jr., Ph.D., Professor of Ethnobotony, Notional University of Mexico Thomas J. Carlson, M.S., M.D., Senior Director, Center for Food Safety and Applied Nutri~on , Food and Drug Administro~on , Washington, D.C. -

The Trillion Dollar Question: Tracking Public and Private Investment in Transport
Working Paper THE TRILLION DOLLAR QUESTION: TRACKING PUBLIC AND PRIVate INVESTMENT IN TRANSport BENOIT LEFEVRE, DAVID LEIPZIGER, MATTHEW RAIFMAN EXECUTIVE SUMMARY CONTENTS In a first step to quantify global public and private invest- ment in transport across all modes, WRI estimated annual Executive Summary............................................................1 capital expenditures (excluding consumer spending) at Introduction........................................................................2 between US$1.4 trillion and US$2.1 trillion annually Investment Estimates .........................................................3 (Figure 1). In aggregate, this investment consists of Conclusions .......................................................................6 slightly more private investment than public. Public investment, at US$569 billion to US$905 billion per year, Appendix ............................................................................8 consists almost exclusively of domestic budget expendi- References ........................................................................12 tures. In 2010, 2 percent of public investment was inter- national, mostly provided through official development assistance (ODA). Less than half a percent comes from Disclaimer: Working Papers contain preliminary climate-focused funds and institutions. Private investment, research, analysis, findings, and recommendations. They including both domestic and cross-border flows, is esti- are circulated to stimulate timely discussion and critical -
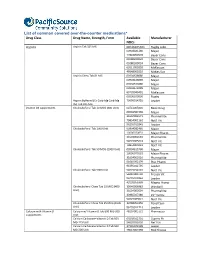
List of Common Covered Over-The-Counter Medications*
List of common covered over-the-counter medications* Drug Class Drug Name, Strength, Form Available Manufacturer NDCs Aspirin Aspirin Tab 325 MG 00536105301 Rugby Labo 00904681180 Major 12843054578 Bayer Cons 00280200020 Bayer Cons 00280200024 Bayer Cons 62011002003 McKesson 49348000110 McKes Sun Aspirin Chew Tab 81 MG 00904628880 Major 00904628889 Major 00904679480 Major 00904679489 Major 63739043401 McKesson 00536100836 Rugby Aspirin Buffered (Ca Carb-Mg Carb-Mg 70000014701 Leader Ox) Tab 325 MG Vitamin D3 supplements Cholecalciferol Tab 10 MCG (400 Unit) 00761005820 Basic Drug 00904582360 Major 31604002671 PharmaVite 79854001162 Nat’l Vit 96295012845 Leader Cholecalciferol Tab 1000 Unit 00904582460 Major 10006070033 Major Pharm 31604002683 PharmaVite 54629005024 Nat’l Vit 79854005023 Nat’l Vit Cholecalciferol Tab 50 MCG (2000 Unit) 00904615760 Major 10006070162 Major Pharm 31604002516 PharmaVite 51645092199 Plus Pharm 96295012795 Leader Cholecalciferol Tab 5000 Unit 54629794101 Nat’l Vit 58487003702 Freeda Vit 96295012846 Leader 43292056338 Magno-Hump Cholecalciferol Chew Tab 10 MCG (400 35046006982 Windwill Unit) 31604002604 PharmaVite 40985027380 21st Centu 54629089821 Nat’l Vit Cholecalciferol Chew Tab 25 MCG (1000 30768050356 Rexall Sun Unit) 96295012713 Leader Calcium with Vitamin D Calcium w/ Vitamin D Tab 600 MG-200 48107005122 Pharmassur supplements Unit Calcium Carbonate-Vitamin D Tab 500 60258012701 Cypress Ph MG-125 Unit 54629031630 Nat’l Vit Calcium Carbonate-Vitamin D Tab 500 37205043198 Leader MG-200 Unit 49614067298 -

Tobacco Control Policy-Making in Portugal: Vested Interests Or Public
Tobacco Prevention & Cessation Industry Monitoring Letter Tobacco control policy-making in Portugal: vested interests or public health? Sofia Ravara1,2, Hilson Cunh Filho2,3, Paula Lobato Faria4, Natercia Miranda2, Jose Manuel Calheiros1,2 INTRODUCTION acceptance of the tobacco industry and interfere 4,14 Tobacco use remains a threat to global health in policy-making . AFFILIATION and socio-economic development1. In order to Currently, the EU has a window of opportunity 1 Faculty of Health Sciences, University of curb the tobacco epidemic, the World Health to advance TC: the implementation of the Tobacco Beira Interior, Covilha, Organisation (WHO) developed the Framework Products Directive (TPD)15. The challenge now Portugal 2 Centre for Advocacy, Convention on Tobacco Control (FCTC), facing EU MS is its transposition into national Treatment and which aims to protect populations from tobacco legislation, effective enforcement and subsequent Reabilitation, NGO(CATR), 2 monitoring. Lisbon, Portugal consumption and exposure to tobacco smoke . 3 Faculty of Social The FCTC binds its ratifying parties, such as The weaknesses of loopholes in tobacco Sciences and Humanities, 4, 16-17 NOVA University of Lisbon, the European Union (EU) and the EU Member control policy-making are well documented . Portugal States (MS), to implement evidence-based Following FCTC ratification by Portugal in 2005, 4 National School of Public 2 Health, NOVA University of tobacco control (TC) policies . Although FCTC a new tobacco law was approved in 2007 and Lisbon, Portugal policies are implementable at the EU and MS came into force in January 200818. Cunha-Filho et CORRESPONDENCE TO level, Europe still ranks first regarding smoking al., using the case of the Portuguese 2007 tobacco Ravara S. -

Petition for a Writ of Mandamus
Case: 18-114 Document: 2-1 Page: 1 Filed: 12/01/2017 (1 of 257) No. 17-______ IN THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT ________________ IN RE AMARIN PHARMA, INC. AND AMARIN PHARMACEUTICALS IRELAND LTD., Petitioners. ________________ On Petition for a Writ of Mandamus to the United States International Trade Commission, In the Matter of Certain Synthetically Produced, Predominantly EPA Omega-3 Products in Ethyl Ester or Re-esterified Triglyceride Form, ITC Docket No. 3247. ________________ PETITION FOR A WRIT OF MANDAMUS ________________ Of Counsel: Ashley C. Parrish Joseph T. Kennedy Principal Counsel Barbara Kurys Jeffrey M. Telep AMARIN PHARMA, INC. Lisa M. Dwyer* 1430 Route 206 Jesse Snyder Bedminster, NJ 07921 KING & SPALDING LLP Telephone: (908) 719-1315 1700 Pennsylvania Ave. N.W. Facsimile: (908) 719-3012 Washington, DC 20006-4706 Telephone: (202) 737-0500 Facsimile: (202) 626-3737 Email: [email protected] Counsel for Petitioners Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. December 1, 2017 *Admission Pending Case: 18-114 Document: 2-1 Page: 2 Filed: 12/01/2017 (2 of 257) CERTIFICATE OF INTEREST Counsel for Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. certify the following: 1. The full name of every party or amicus represented herein: Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. 2. The name of the real party in interest (if the party named in the caption is not the real party in interest) represented us: Not applicable. 3. All parent corporations and any publicly held companies that own 10 percent or more of the stock of the party or amicus curiae represented herein: Amarin Pharma, Inc. -

SHORT REPORT Influenza Season 2012–2013 in Europe: Moderate
Epidemiol. Infect. (2014), 142, 1809–1812. © European Centre for Disease Prevention and Control (ECDC) 2014 doi:10.1017/S0950268814001228 SHORT REPORT Influenza season 2012–2013 in Europe: moderate intensity, mixed (sub)types R. SNACKEN1*, E. BROBERG1,J.BEAUTÉ1,J.E.LOZANO2,P.ZUCS1 1 AND A. J. AMATO-GAUCI on behalf of the European Influenza Surveillance Network 1 European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden 2 Public Health Directorate General, Regional Ministry of Health. Government of Castilla y León, Valladolid, Spain Received 30 October 2013; Final revision 21 March 2014; Accepted 23 April 2014; first published online 9 May 2014 SUMMARY This paper summarizes influenza activity in the European Union/European Economic Area (EU/EEA) in 2012–2013. The influenza season 2012–2013 in Europe lasted from early December to late April. Overall the severity of the season could be described as moderate, based on the ILI/ARI consultation rates and the percentage of sentinel specimens positive for influenza compared to previous seasons. Both influenza A and B viruses circulating accounted for 47% and 53% of positive sentinel specimens, respectively, with both A(H1) and A(H3) varying for dominance. Compared to outpatients, the proportion of laboratory-confirmed influenza hospitalized cases infected by A(H1N1)pdm09 was significantly higher in middle-aged patients (33% vs. 17%, χ2 =66·6, P<0·01). Despite a relatively good match between vaccine and circulating strains, vaccine effectiveness was estimated to be moderate. Key words: -

Agriculture and Environment
Agriculture and environment Contents 1. Agriculture and INPUT USE .................................................................................................................................................................................................................. 5 Farming intensity ........................................................................................................................................................................................................................................ 5 Livestock density ........................................................................................................................................................................................................................................ 6 Energy use in agriculture ............................................................................................................................................................................................................................ 7 Energy use in the food industry .................................................................................................................................................................................................................. 8 Production of renewable energy ................................................................................................................................................................................................................. 9 Fertilizer consumption ............................................................................................................................................................................................................................. -

Understanding the Socio-Economic Divide in Europe
UNDERSTANDING THE SOCIO-ECONOMIC DIVIDE IN EUROPE 26 January 2017 UNDERSTANDING THE SOCIO-ECONOMIC DIVIDE IN EUROPE BACKGROUND REPORT 26 JANUARY 2017 ACKNOWLEDGEMENTS This background report is supported by a grant from the Open Society Foundations. This report has been prepared under the umbrella of the OECD Centre for Opportunity and Equality (COPE). It has been prepared and coordinated by the Social Policy Division (Michael Förster, Ana Llena Nozal and Céline Thévenot). The report is based on inputs from the International Migration Division (Thomas Liebig and Cécile Thoreau), the Health Division (Chris James and Gaetan Lafortune) and the Employment Division (Paolo Falco, Pascal Marianna). Technical assistance was provided by Nathalie Bienvenu, and statistical assistance was provided by Maxime Ladaique, both from the Social Policy Division. Authors are grateful to Stefano Scarpetta, (Director for Employment, Labour and Social Affairs ELS), Mark Pearson (Deputy Director, ELS,) and Shruti Singh (Manager, COPE) for their comments. This document is available online via http://oe.cd/cope-divide-europe-2017. The opinions expressed and arguments employed herein do not necessarily reflect the official views of OECD member countries or the European Union. This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of international frontiers and boundaries and to the name of any territory, city or area. Throughout this document, the OECD, OED-EU and EU averages refer to the unweighted average for the relevant countries for which data are available. (↘) (or ↗) in the legends relate to the variable for which countries are ranked from left to right in decreasing (or increasing) order. -

Natural Medications for Psychiatric Disorders
Natural Medications for Psychiatric Disorders David Mischoulon, MD, PhD Director Depression Clinical and Research Program Massachusetts General Hospital Associate Professor of Psychiatry Harvard Medical School www.mghcme.org Disclosures • My spouse/partner and I have the following relevant financial relationship with a commercial interest to disclose: –Research support from Nordic Naturals –Unpaid consulting for Pharmavite, LLC, and Gnosis, Inc. –Royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives” www.mghcme.org 2 Objectives • To understand the evidence base for efficacy of natural therapies in psychiatry • To identify the risks and benefits of various natural treatments in psychiatry • To be able to educate patients in purchasing natural products in both over- the-counter and prescription forms www.mghcme.org Pros and Cons of Natural Remedies • In 2007, 38% of adults and 12% of children used CAM practices and products in the past year (NIH, 2010) – about $33.9 billion out-of-pocket cost • Easy access, good tolerability • Used by many who don’t respond to standard therapies • Limited research/systematic studies • “Natural” does NOT mean “safe” • Toxicity, adverse effects, interactions • Different preparations/purity • Insurance does not cover them www.mghcme.org 5 St. John’s Wort (SJW, Hypericum Perforatum) www.mghcme.org 6 St John’s Wort • About 40 published trials; many comparisons with TCAs and SSRIs; various systematic reviews and meta-analyses -

Nordic Alcohol Policy in Europe: the Adaptation of Finland's, Sweden's and Norway's Alcohol
Thomas Karlsson Thomas Karlsson Thomas Karlsson Nordic Alcohol Policy Nordic Alcohol Policy in Europe in Europe The Adaptation of Finland’s, Sweden’s and Norway’s Alcohol Policies to a New Policy Framework, 1994–2013 The Adaptation of Finland’s, Sweden’s and Norway’s Alcohol Policies to a New Policy Framework, 1994–2013 Nordic Alcohol Policy in Europe Policy Alcohol Nordic To what extent have Finland, Sweden and Norway adapted their alcohol policies to the framework imposed on them by the European Union and the European Economic Area since the mid-1990s? How has alcohol policies in the Nordic countries evolved between 1994 and 2013 and how strict are their alcohol policies in comparison with the rest of Europe? These are some of the main research questions in this study. Besides alcohol policies, the analyses comprise the development of alcohol consumption and cross-border trade with alcohol. In addition to a qualitative analysis, a RESEARCH quantitative scale constructed to measure the strictness of alcohol policies is RESEARCH utilised. The results from the study clearly corroborate earlier findings on the significance of Europeanisation and the Single Market for the development of Nordic alcohol policy. All in all, alcohol policies in the Nordic countries are more liberal in 2013 than they were in 1994. The restrictive Nordic policy tradition has, however, still a solid evidence base and nothing prevents it from being the base for alcohol policy in the Nordic countries also in the future. National Institute for Health and Welfare P.O. -

Land Cover Fire Proneness in Europe Mario G
Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) Forest Systems 2014 23(3): 598-610 http://dx.doi.org/10.5424/fs/2014233-06115 ISSN: 2171-5068 eISSN: 2171-9845 OPEN ACCESS Land cover fire proneness in Europe Mario G. Pereira1,2*, Jose Aranha1 and Malik Amraoui2,3 1 Centro de Investigación e de Tecnologias Agro-Ambientais e Biológicas, CITAB, Universidade de Trás-os-Montes e Alto Douro, UTAD, Quinta de Prados, 5000-801 Vila Real, Portugal. 2 Instituto Dom Luiz (IDL), University of Lisbon. Lisbon, Potugal. 3 Universidade de Trás-os-Montes, UTAD, Quinta de Prados, 5000-801 Vila Real, Portugal Abstract Aim of study: The characterization of the fuels is an important aspect of the fire regime in each specific ecosystem while fire is an important disturbance for global vegetation dynamics. This study aims to identify and characterize the spatial and temporal evolution of the fire incidence and of the vegetation types that are most affected by forest fires in Europe, with emphasis on the mixed forests. Area of study: Europe. Material and methods: Corine Land Cover maps for 2000 and 2006 (CLC2000, CLC2006) and burned area (BA) perimeters, from 2000 to 2013 in Europe are combined to access the spatial and temporal evolution of the types of vegetation that are most affected by fires using geostatistics and Geographical Information System (GIS) techniques. Main results: The spatial and temporal distribution of BA perimeters, vegetation and burnt vegetation by fires was performed and different statistics were obtained for Mediterranean and northern Europe, confirming the usefulness of the used land cover classification.